A tiny oxygen generator that can be implanted directly into the hypoxic center of tumors using a biopsy needle has been unveiled by researchers in the U.S. The implantable micro-oxygen generator (IMOG) could potentially improve the efficacy of radiotherapy.
The generator uses on-board electronics to convert external ultrasound signals into a small DC voltage, which in turn generates oxygen in situ via water electrolysis. Initial trials described in an article published online August 4 in IEEE Transactions on Biomedical Engineering have shown that the IMOG can raise oxygen concentration in hypoxic areas to normal levels in just 10 minutes.
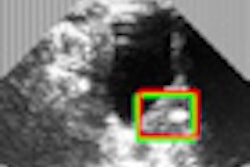
 Researchers have created and tested a miniature device, seen here, that can be implanted in tumors to generate oxygen, boosting the killing power of radiation and chemotherapy. The technology is designed to treat solid tumors that are hypoxic at the center. The device (right) fits inside a tube (left) that can then be inserted into a tumor with a biopsy needle. Image credit: Birck Nanotechnology Center, Purdue University.
Researchers have created and tested a miniature device, seen here, that can be implanted in tumors to generate oxygen, boosting the killing power of radiation and chemotherapy. The technology is designed to treat solid tumors that are hypoxic at the center. The device (right) fits inside a tube (left) that can then be inserted into a tumor with a biopsy needle. Image credit: Birck Nanotechnology Center, Purdue University.
Device design
Measuring just 1.2 x 1.3 x 8 mm3, the IMOG comprises four main components: an ultrasonic receiver, rectifying circuitry, electrodes, and an ion-exchange membrane. Once the IMOG is implanted in the tumor, the team transmits an ultrasound signal through the patient that excites the ultrasonic receiver onboard the IMOG and generates an AC voltage. The rectifying circuitry converts this to a DC voltage, which is then applied to the electrodes to separate oxygen from hydrogen in water.
"The ion-exchange membrane plays an important role in the successful operation of the IMOG," Ziaie told medicalphysicsweb. "Integrating such a small ion-exchange membrane was a challenge, but it was crucial. Performing water electrolysis without the membrane would create caustic soda and chlorine gas, both of which are harmful. The ion-exchange membrane prevents this reaction from taking place."
To ensure biocompatibility, the vast majority of the device is encapsulated in parylene. Only the small section containing the electrodes and the ion-exchange membrane is exposed to the surrounding tissue to gain direct access to the water in the tumor.
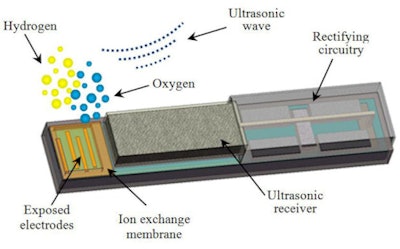 |
| This diagram shows the design of a miniature device that can be implanted in tumors to generate oxygen, boosting the killing power of radiation and chemotherapy. Image credit: Birck Nanotechnology Center, Purdue University. |
Initial tests
In collaboration with Dr. Song-Chu Ko, PhD, an assistant professor of clinical radiation oncology at Indiana University School of Medicine, Ziaie and his team at Purdue have performed a series of tests to characterize the performance of the IMOG device.
For example, the researchers have identified the optimal excitation ultrasound frequency and studied the IMOG output voltage versus the distance from the ultrasound transmitter at different angles. Although the output voltage drops exponentially with distance, there was little angular dependence. This finding is particularly important as multiple IMOGs could be inserted into larger tumors without having to worry about their orientation to the ultrasound transmitter.
Following the success of these tests, the team analyzed the performance of the IMOG in vivo by monitoring oxygen levels in the BxPC-3 pancreatic tumor model grown in the flanks of athymic mice. Three mice, each with two tumors, were involved in the study. In each case, one of the tumors was used as a control while the other was oxygenated by the IMOG.
"The IMOG was capable of oxygenating the tumors in less than 10 minutes," said Ziaie. "We did not notice any oxygen change in the tumors without the IMOG. The IMOG was found to be operational two weeks after implantation."
The next milestone on the horizon will be clinical trials, which the team hopes to perform in 2013. In practice, the IMOG will remain implanted in the tumor when a patient undergoes radiotherapy.
A patent application has also been filed. Future work may also focus on redesigning the device to make it more practical for manufacturing.
© IOP Publishing Limited. Republished with permission from medicalphysicsweb, a community website covering fundamental research and emerging technologies in medical imaging and radiation therapy.