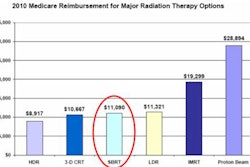
The Advanced Medical Technology Association (AdvaMed) and the Medical Imaging and Technology Alliance (MITA) have announced an industrywide effort to develop and implement additional patient protection features for radiation therapy (RT) equipment.
The announcement was made in conjunction with a two-day meeting being held this week by the U.S. Food and Drug Administration (FDA) to discuss ways that therapeutic devices can be made safer to prevent radiation therapy errors.
Titled the Radiation Therapy Readiness Check Initiative, the project is intended to add features to equipment that confirm that patient treatment plans are delivered as intended. The program will also ensure that radiation therapy equipment, accessories, and patients are properly positioned prior to delivery of therapy, according to a statement issued jointly by the Washington, DC, associations.
Radiation therapy manufacturers affiliated with AdvaMed and MITA have agreed to begin immediately developing and incorporating the safety features into new products. They will also have them available within two years for compatibly configured products already installed in cancer treatment centers.
The initiatives specifically include:
- Smart software in radiation therapy equipment that will not enable systems to function until the quality assurance program for an individual patient's treatment program has been completed and verified, and designated approval authorization has been recorded.
- An enhanced beam-modification check to verify the correct placement of appropriate beam-modifying accessories such as wedges, blocks, and compensators. If accessories are incorrect, missing, or improperly placed, the RT system will not operate.
- A patient positioning confirmation system in which enhancements to the RT system will generate a visual representation of the planned relative positions of the patient. The treatment device will allow its operator to quickly compare the patient's actual position with the visual representation. The radiation therapist also will have to enter a confirmation of a positive comparison.
The FDA meeting was held in Washington, DC, on June 9-10 to solicit radiation therapy practitioners, manufacturers of radiation therapy equipment, professional organizations representing both groups, and the general public to discuss steps that could be taken to help reduce misadministration, misaligned exposures, and other adverse events relating to the use of RT equipment.
By Cynthia E. Keen
AuntMinnie.com staff writer
June 10, 2010
Related Reading
Rad therapy groups support FDA review, April 13, 2010
FDA turns attention to radiation therapy devices, April 8, 2010
FDA hearings rise above medical radiation rhetoric, March 31, 2010
Copyright © 2010 AuntMinnie.com