Biology-guided radiotherapy looks promising for concurrent monitoring of both anatomical and metabolic tumor response, particularly in lung cancer cases, according to initial results presented June 23 at the 2025 Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
Through real-time PET signal monitoring, the novel radiation treatment system Scintix enabled successful dose delivery for a small group of lung and bone cancer patients, while managing inter- and intrafraction tumor motion, according to nuclear medicine physicist Rameshwar Prasad, PhD, who led the research at UT Southwestern Medical Center in Dallas, TX, and presented early findings.
"In conventional radiation therapy, which relies mostly on CT or MRI imaging and PET also, if we take these images into planning consideration, this does not account for any accurate tumor motion and biology when the treatment is going on," Prasad said.
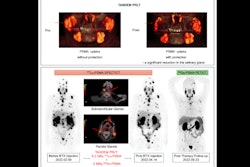
Toward adaptive radiation therapy, the system called Scintix refers to scintigraphy or scintillation events, which is the process of using radiotracers or PET detectors to localize and monitor, while X refers to treating with megavoltage (MV) energy x-rays or photons, according to Prasad.
The platform features a rotating ring gantry (60 rpm) system equipped with a 6MV S-band linear accelerator, two opposed PET detector arc arrays, a 64-leaf binary multileaf collimator, an MV imaging panel, and a fan-beam CT scanner.
As a potential alternative to standard radiation therapy, the fludeoxyglucose F-18-guided system is intended to detect and process tumor PET emissions in real-time so that a patient's radiation dose is effectively tracked to lesion motion, using the biology-guided radiotherapy (BgRT) device RefleXion.
During his talk, Prasad correlated planning target volume (PTV) with BgRT margin concepts, such biology tracking zone (BTZ) that represents volume for masking PET signals outside the tumor region of interest, net target activity concentration (AC) in the BTZ and normalized target signal (NTS), and how AC and NTS thresholds signal the system to allow treatment.
In a study, Prasad and colleagues evaluated 27 patients for BgRT. Using the system's automated functions, only 14 could be treated. They received a total of 54 fractions, all delivered successfully as they were planned, Prasad reported.
PET activity was calibrated using a uniform phantom to convert counts per second (cps) to becquerel (Bq) for F-18- and gallium-68 (Ga-68)-based radiopharmaceuticals. Simultaneous quantitative PET imaging demonstrated a correlation between tumor size reduction and FDG uptake.
The team could see AC in the tumor going down relative to each fraction, and they observed that same trend for the NTS, Prasad said, emphasizing the importance of being able to adapt at each fraction.
System calibration showed 2% error for F-18 (expected: 6.44 kBq/mL, measured: 6.31 kBq/mL) and <1% for Ga-68, according to the results.
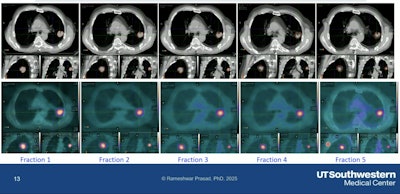 Tumor changes observed at each fraction in the adaptive system.Rameshwar Prasad, PhD, UT Southwestern Medical Center, and SNMMI
Tumor changes observed at each fraction in the adaptive system.Rameshwar Prasad, PhD, UT Southwestern Medical Center, and SNMMI
The system was also able to optimize radiation dose to target PET avidity in the tumor. Time to treat ranged from 15 minutes to 45 minutes, depending on the size of the tumor and its location, Prasad said.
"We are working toward adaptive radiation therapy, plus personalized therapy using the PET scanner," Prasad noted. "This has tremendous potential to 'dose-paint' in real time on the same system; however, we need more trials and continued work."
In November 2023, the first patient was treated using the system. Ongoing research will further evaluate long-term clinical outcomes and potential impact on treatment efficacy across various tumor types.