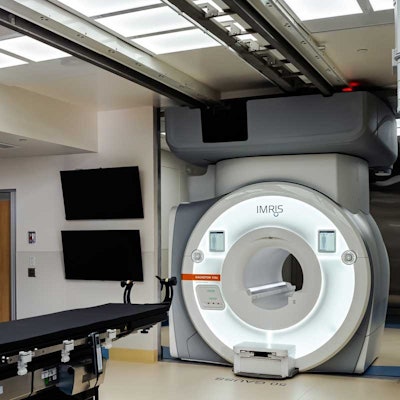
An IMRIS ceiling-mounted diagnostic and intraoperative MRI system.
IMRIS
IMRIS has received clearance from the U.S. Food and Drug Administration (FDA) for its InVision 3T Recharge MRI system.
The ceiling-mounted diagnostic and intraoperative MRI system provides a path for IMRIS Operating Suites currently using Magnetom Verio scanners from Siemens Healthineers to upgrade to the company’s Magnetom Skyra Fit scanners. InVision 3T Recharge brings Siemens’ latest Skyra Fit Biomatrix technology to IMRIS Operating Suites, which extends the life of the initial investment and enhances the value of MRI at these clinical centers, the company said.
IMRIS custom designs intraoperative MRI systems specifically for imaging during neurosurgical procedures, according to the firm.