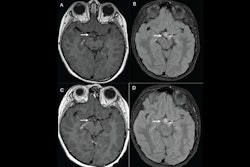
Adverse reactions to gadolinium-based contrast agents (GBCAs) in children occur at low rates, researchers have reported in a literature review.
The review suggests GBCAs are relatively safe for children -- a population for which clinicians should exercise special concern when it comes to contrast agents, wrote a team led by Ok Jeong Yu, MD, of the University of Ulsan College of Medicine in Seoul, South Korea. The paper was published July 29 in Radiology.
"[Our review found that] gadolinium-based contrast agent-associated adverse reaction rates in children undergoing contrast-enhanced MRI were low for acute physiologic reactions, acute allergic-like reactions, and delayed reactions, with no nephrogenic systemic fibrosis reported," the group noted.
Despite previous studies that show low adverse reactions to GBCAs among children, safety concerns persist, the investigators wrote. They conducted a literature review to evaluate the safety of the agents in children and adolescents undergoing contrast-enhanced MRI exams, focusing on GBCA-associated adverse drug reactions, nephrogenic systemic fibrosis (NSF), and gadolinium deposition in the brain.
The authors searched for studies from MEDLINE/PubMed, Embase, Web of Science, and the Cochrane Library published up to November 2023. Of these studies, 32 covered 112,760 exams that addressed GBCA-related adverse drug reactions; eight covered 4,895 exams that addressed nephrogenic systemic fibrosis; and six covered 787 exams that addressed brain gadolinium deposits. The researchers pooled studies that reported incidences of GBCA-associated adverse drug reactions with information on GBCA type and used a linear mixed-effects model to assess gadolinium deposits in the brain.
Overall, they reported the following:
- The acute GBCA-associated adverse drug reaction rate was 0.25% for physiologic reactions and 0.13% for allergic reactions.
- The delayed adverse drug reaction rate was 0.04%.
- No patients were reported with nephrogenic systemic fibrosis.
In an accompanying commentary, Ilya Abelev, MD, and Matthew McInnes, MD, both of Ottawa Hospital in Ontario, Canada, noted that the "absence of NSF in this large pediatric cohort, including in patients with renal insufficiency, is a particularly reassuring finding" -- but they also urged more research.
"As stewards of pediatric imaging, radiologists must continue to balance the necessity of precise diagnostic information against potential safety concerns, always prioritizing patient welfare," they wrote. "Ongoing research, adherence to established guidelines, and transparent communication will be crucial as we advance our understanding and refine our practices. Ultimately, studies such as this serve not only to inform evidence-based clinical practice but also to reinforce the importance of patient-centered care in radiology."
The complete research can be found here.