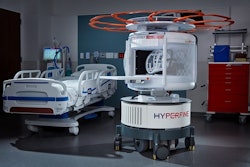
The U.S. Food and Drug Administration (FDA) has cleared new AI software for Hyperfine’s Swoop portable MR brain imaging system.
This is the ninth generation of AI-powered Swoop system software. The updated software enables faster acquisition of ultralow field brain MR images without affecting image quality, which could help in treating time-sensitive medical conditions such as stroke, according to Hyperfine.