Risks associated with gadolinium-based contrast agents (GBCAs) differ by drug, and clinicians would do well to educate themselves about these differences, according to research published October 14 in Expert Opinion on Drug Safety.
To make the situation more complicated, not all possible adverse events from GBCAs are noted in drug packaging, wrote a team led by Lu Wang, PhD, of Yantai Yuhuangding Hospital in Yantai, China.
"Some adverse events [noted] in this study were not mentioned in the package inserts, which need more attention and research to ensure the clinical safety," the group explained.
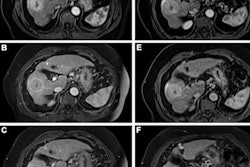
The U.S. Food and Drug Administration (FDA) approved the first GBCA in 1988, and ever since, this class of drugs has been used with MRI to diagnose disease. GBCAs are categorized as either macrocyclic or linear; macrocyclic agents are considered more stable. Adverse drug reactions to GBCAs have been reported in recent years, ranging from allergic responses, nephrogenic systemic fibrosis, kidney injury, and gadolinium deposits, the team noted.
Wang and colleagues sought to investigate the "risk signals" of various GBCAs (that is, information that points to risk of adverse effects from the agent). They investigated these indicators prompted by the following four GBCAs between 2004 and 2022, using the U.S. Food and Drug Administration's (FDA) Adverse Event Reporting System:
- Gadopentetate dimeglumine (Gd-DTPA) -- 6,813 cases
- Gadobenate dimeglumine (Gd-BOPTA) -- 3,142 cases
- Gadoteridol (Gd-HP-DO3A) -- 1,507 cases
- Gadobutrol (Gd-BT-DO3A) -- 2,800 cases
The investigators assessed the risk signals using reporting odds ratio and proportional reporting ratio; they identified 424 risk signals in the four agents:
| Number of risk signals by type of GBCA |
|
|---|---|
| Type |
Number |
| Gd-DTPA |
151 |
| Gd-BOPTA |
93 |
| Gd-HP-DO3A |
79 |
| Gd-BT-DO3A |
101 |
The risk signals involved 20 system organ systems; two of the top four of these were "skin and subcutaneous tissue disorders" and "general disorders and administration site conditions," but adverse events included NSF, urticaria, gadolinium deposits, dizziness, headache, and vomiting. Adverse events occurred more frequently among women across all four drugs. The group noted that some adverse events that occurred in the study population were not mentioned in the drug package inserts.
More research on the effects of GBCAs is needed, according to the authors.
"Although most of [adverse events caused by] GBCAs are mild, there are still some life-threatening [ones]," they concluded. "Extra studies are needed to enhance the safety of GBCAs."
The complete study can be found here.