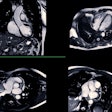
The U.S. Food and Drug Administration (FDA) has cleared Insight Medbotics' Image Guided Automated Robot system.
The system is the first and only robotic guidance and placement system cleared to work inside an MRI bore, one of the most challenging hospital environments, and it is indicated for image-guided breast biopsies, the company said.
The IGAR system can be mounted on an MRI table, where it automatically executes the clinical plan for biopsies, and this may increase physician autonomy while standardizing skill requirements and reducing burden on staff, Insight said.
The robotic system was validated last year in a study published in the International Journal of Medical Robotics and Computer Assisted Surgery, the company noted.