Japanese researchers have found that mice that were given gadolinium-based contrast agents (GBCAs) typically used in MRI scans did not show any motor or behavioral brain changes, according to a study published August 31 in Radiology.
The findings could translate to a better understanding of how GBCAs affect people, wrote a team led by Dr. Hiroyuki Akai, PhD, of the University of Tokyo in Japan.
"Our findings may also partially reduce health concerns [about] gadolinium deposition in the brain," the group wrote.
Gadolinium contrast is considered relatively safe for people with normal kidney function, but the fact remains that the element is toxic, the group noted. Recent studies have revealed that trace elements of gadolinium remain in the body -- particularly the brain -- after MRI scans, but it's not clear whether these deposits have any clinical significance. Macrocyclic GBCAs tend to be more stable than linear ones, and gadolinium deposits in the brain are more common after patients undergo injection of linear GBCAs.
Still, deposition of gadolinium in the brain is a clinical concern, and its effect needs more research, Akai and colleagues wrote. To further investigate any motor or behavioral changes after recurring injection of GBCA, the group conducted an animal study with mice.
Over the course of eight weeks, three groups of 17 mice were injected twice per week with the following:
- Group A (control): Saline
- Group B: Macrocyclic GBCA
- Group C: Linear GBCA
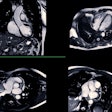
The mice underwent brain MRI exams every other week to identify any signal intensity changes caused by gadolinium deposits. The researchers tracked the animals' motor ability, anxiety levels, and memory through various tests (i.e., rotarod performance, open field, plus-maze, light-dark anxiety, locomotor activity assessment, passive avoidance memory, and Y-maze).
Akai et al found gadolinium deposits in the bilateral deep cerebellar nuclei in mice who received the linear GBCA. But further analysis did not show any behavioral, anxiety level, or long- or short-term memory changes in either these mice or those that were injected with the macrocyclic GBCA.
"[Our] findings agree with findings from previous rat studies and, to our knowledge, our study is the first to clarify this in mice by using MRI," the group noted.
The results may help mitigate concerns about gadolinium deposits in the brain, but they don't necessarily translate to clinical use of linear GBCA in people, the team cautioned.
"Although our study simulates gadolinium deposition in humans and no apparent motor behavioral changes were observed ... our findings do not directly support the clinical use of linear GBCA," the authors wrote. "In particular, concern about nephrogenic systemic fibrosis in patients with decreased renal function strongly restricts the use of linear GBCA."
In any case, animal studies can provide important data when it comes to thorny clinical questions such as the effect of GBCA on the brain, wrote Dr. John Chen, PhD, of Massachusetts General Hospital in Boston in an accompanying editorial.
"There are still gaps in our knowledge regarding GBCA deposition in the brain for which animal studies could elucidate," he wrote.