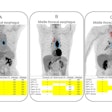
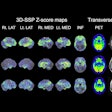
The U.S. Food and Drug Administration (FDA) has granted fast-track designation to Telix Pharmaceuticals’ PET radiotracer F-18 floretyrosine (Pixclara) for imaging progressive or recurring gliomas.
Pixclara was developed by researchers at the University of California, San Francisco. The tracer reveals the activity of specific transporter proteins known as LAT1 and LAT2, which are highly active in brain regions with gliomas. The technique can help determine if a glioma is truly progressing or undergoing a treatment-induced change, known as pseudo-progression, where standard MRI is often inconclusive, the company said.
Pixclara previously has been granted orphan drug designation in the U.S. and Telix is preparing a New Drug Application (NDA) for its use in both adult and paediatric patients, the company said. The fast-track designation enables expedited review and closer consultation with the FDA during the review process.
Telix has selected PharmaLogic Holdings as its commercial manufacturing and pharmacy distribution partner, the company added.