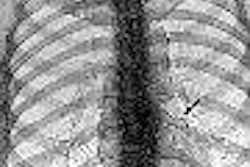
Analogic has received 510(k) clearance from the Food and Drug Administration for the GR17 flat-panel digital detector developed by Analogic subsidiary Anrad.
GR17 is designed for use in all general radiography applications and is the fifth in a series of high-resolution amorphous selenium digital detectors produced by Anrad. Its 17-inch-square active area provides for optimum exposure coverage and simplifies positioning the detector around the patient, according to the Peabody, MA-based company.
By AuntMinnie.com staff writers
November 1, 2004
Related Reading
Analogic to delay its annual 10-k report, November 2, 2004
Analogic delays 10-K filing, October 15, 2004
Analogic selects Burch as VP, October 1, 2004
Analogic Q4 revenues surge, September 22, 2004
Analogic taps Harris as security VP, September 16, 2004
Copyright © 2004 AuntMinnie.com