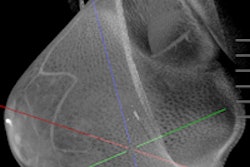
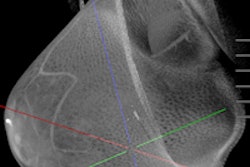
Breast CT developer Koning has received U.S. Food and Drug Administration (FDA) approval for its breast CT scanner, including a biopsy bracket add-on.
Koning completed the FDA's premarket approval (PMA) process for the 3D breast CT scanner, as well as an optional biopsy bracket for CT-guided biopsies of suspicious breast lesions. Other optional accessories for the system include a collimator that limits the x-ray beam to the area of interest, according to the vendor.
More than 680 breast CT scans were performed at Elizabeth Wende Breast Care in Rochester, NY, and the University of Rochester Medical Center, with additional collaboration at the University of Massachusetts Medical Center in Worcester, MA, Koning said. A large reader study led by Dr. Etta Pisano was also conducted at the Medical University of South Carolina.