CT screening of high-risk patients detects many smaller early stage lung cancers, according to a study by Mayo Clinic researchers. But the data does not suggest a mortality benefit, and it is also unclear if the findings represent a true stage shift, the group concluded after a five-year prospective study of 1,520 current and former smokers, both men and women.
"CT screening for lung cancer offers the possibility of reducing mortality from lung cancer. Our preliminary results do not support this possibility, and may raise concerns that false-positive results and overdiagnosis could actually result in more harm than good," wrote Dr. Stephen J. Swensen and colleagues from the Mayo Clinic in Rochester, MN (Radiology, February 4, 2005).
Five annual CT exams were conducted as part of the study, and 3,356 uncalcified lung nodules were identified in 1,118 (74%) patients. And 68 lung cancers were diagnosed in 66 patients, with two of the cancers detected through sputum cytology.
On the downside, rates of false-positive findings ranged from 92.4% to 96.0%. There was at least one false-positive finding in 69% of the participants. The overall lung cancer mortality rate was 1.6 per 1,000 person years.
To compare their results with past observations, the researchers used the findings of the Mayo Lung Project from the 1970s (Journal of the National Cancer Institute, August 2000, Vol. 92:16, pp. 1308- 1316).
The researchers found no difference in the incidence lung cancer mortality rates between the two studies, and no difference in the percentage of patients diagnosed with stage I non-small cell lung cancers, including carcinoma in situ.
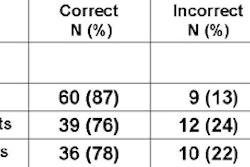
Detection of early stage lung cancers
Scanning was done on a four-slice helical CT scanner (LightSpeed QX/i; GE Medical Systems, Chalfont St. Giles, U.K.) with a 5-mm section width with 3.75-mm reconstruction interval, high-speed mode, pitch of 1.5, exposure time of 0.8 seconds per rotation, table feed of 30 mm per rotation of 37.5 mm/sec, 120 kVp, and 40 mA. Effective radiation dose was 0.65 mSv. Scans were obtained from the level of the sternal notch to the iliac crests.
Nodules identified in the baseline year, when the first CT screening exam was conducted, were classified as prevalence nodules. Any prevalence nodule diagnosed as cancer was termed prevalence cancer.
All nodules identified in the subsequent four annual CT examinations were considered incidence nodules and the cancers as incidence cancers.
For the purpose of analysis and comparison, the incidence cancers were grouped along with cancers that were discovered in the intervening periods between the annual screenings through interval scans.
"Diagnosis of cancer was assigned with histologic or cytologic findings in all cases. Staging was determined at surgery, if performed, and according to supportive biopsy results as available (or by means of PET and/or CT in the absence of other data)," the researchers stated. Immediate cytologic analysis was done using induced sputum samples obtained on the days of the CT exams.
Of the 3,356 uncalcified nodules that were identified, 61% of nodules were smaller than 4 mm, 31% of nodules were 4-7 mm, 8% of nodules were 8-20 mm, and less than 1% of nodules were larger than 20 mm.
Prevalence non-small cell lung cancers ranged in size from 5-47 mm, and incidence non-small cell lung cancers ranged from 5-50 mm. Of the 28 non-small cell lung cancer incidence cases detected, 17 were stage I tumors.
There was no difference in the percentage of patients diagnosed with stage I non-small cell lung cancers, including carcinoma in situ, the researchers reported. "There was, however, a substantially larger proportion of stage IA cancers detected with CT versus chest radiography in this subset analysis," they noted.
Comparison with Mayo Lung Project
To facilitate a fair comparison between the Mayo Lung Project and the current study, only men age 50 and older were included in the comparison subset, and only the first four years of follow-up were included from the Mayo Lung Project.
Poisson regression and Fischer exact tests were used for the comparison. "There was no difference in the incidence lung cancer mortality rates between the two studies in the subset of men 50 years of age and older with four years of follow-up (2.8 versus 2.0 per 1,000 person years; Poisson regression, p = 0.43)," the researchers noted.
For the stage distribution, there was no difference in the percentage of patients diagnosed with stage I non-small cell lung cancers, including carcinoma in situ (47% versus 41%; Fisher exact test, p = 0.78), they wrote. "There was, however, a substantially larger proportion of stage IA cancers detected with CT versus chest radiography in this subset analysis (47% versus 21%; Fisher exact test, p = 0.05)."
They further observed that to demonstrate a true stage shift, the results should show an increase in early stage disease, as well as a corresponding decrease in late-stage disease when compared with an unscreened population. Similarly, with regard to the mortality estimates, they stated that the observations were consistent with estimates of lung cancer mortality from CT trials.
However, given the study limitations and the shortcomings of using the Mayo Lung Project for comparison, the research group stated that the results "should be viewed as exploratory and hypothesis-generating rather than definitive."
The current study is a single-arm cohort study rather than a randomized one. And since follow-up was only for four years, the early detection benefits may not be evident, the group wrote.
Also, the number of lung cancer deaths observed in the current study could be due to improved detection and diagnosis of lung cancer as the cause of death. In the Mayo Lung Project on the other hand, some lung cancer deaths may have been misattributed to other causes, the researchers stated.
"Before the National Lung Screening Trial (NLST) is completed, screening should be performed in the setting of a clinical trial, or only after informed consent is obtained from a fiduciary without financial interest," the researchers recommended.
Results from the NLST, a randomized controlled trial to determine whether there is a disease-specific mortality benefit from screening, are expected about 2010. The NLST is one of the multicenter clinical trials by the American College of Radiology Imaging Network that relate to cancer diagnosis and treatment (Radiology, June 2003, Vol. 227:3, pp. 631-632).
By N. Shivapriya
AuntMinnie.com staff writer
March 17, 2005
Related Reading
Do whole-body CT's costs outweigh the benefits? February 2, 2005
Lung cancer screening spots lesions early, gives patients additional longevity, December 6, 2004
Diagnostic work-up of CT-detected lung cancer variable, November 22, 2004
Smaller stage I non-small cell lung cancers more curable than larger tumors, September 20, 2004
Copyright © 2005 AuntMinnie.com