Finnish mammography firm Instrumentarium will offer a glimpse next week into its vision of digital mammography's future, unveiling the company's new Diamond interventional breast imaging system at the National Conference on Breast Cancer (NCBC) in San Francisco.
Diamond, which was submitted to the Food and Drug Administration for 510(k) clearance in March, features a charge-coupled device (CCD) detector for digital spot imaging. A prototype of the system, which features a flat-panel amorphous selenium detector, will also be shown.
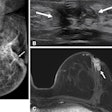
If the FDA approves the product, Diamond will be the first Instrumentarium system to employ the vendor's tuned-aperture computed tomography (TACT) technology. TACT, which has been demonstrated as a work-in-progress at previous RSNA meetings, provides dynamic 3-D breast imaging capability, according to the firm.
With TACT, seven 2-D image slices are obtained from different angles during a standard stereotactic x-ray tube sweep. The images can then be viewed on a workstation in slices, and suspicious regions can be reconstructed into a 3-D image, according to the company, which maintains U.S. operations in Milwaukee.
"Our approach is essentially cost-effective CT of the breast," according to Don Blomstrom, vice president of U.S. mammography sales.
Diamond will ultimately be deployed in concert with an amorphous selenium flat-panel full-field digital detector. For now, however, neither the detector nor supporting technologies such as archiving and display have evolved enough to support full-field digital mammography, Blomstrom said.
"It's our belief that digital mammography will not be deployed (initially) for digitizing the entire breast, but instead will be used just for digitizing suspicious areas of the breast," Blomstrom said.
Once Instrumentarium is able to improve detector yields and take advantage of advances in display and archiving technology to support soft-copy review, it will apply for regulatory clearance for the full-field system, according to Michael Palazzola, president of mammography and dental operations in the U.S. and Latin America. Instrumentarium hopes to submit either a 510(k) or a premarket approval application to the FDA by 2001.
"We could submit for 510(k) clearance right now, but all that existing technology allows you to do is print to hard copy," Palazzola said. "Until there's a 2K x 2K monitor that can last more than 60 days, and you can stack four monitors to allow for review of comparison studies, there's no sense (in) doing this. You have to improve the standard of care."
At present, Diamond's CCD detector only supports 3-D imaging of suspicious regions. The ability to image the entire breast in three dimensions will occur following introduction of the flat-panel detector, which Instrumentarium is sourcing from an undisclosed company.
For a time, Instrumentarium was testing an amorphous silicon detector, but decided to go with amorphous selenium due to its higher detective quantum efficiency (DQE) and lower dose, Blomstrom said.
Along with Diamond's digital imaging benefits come new features such as automated placement of photo cells under the densest part of the breast. Diamond was also designed to easily accommodate interventional procedures, by allowing the system to "park" its tube-head back when not in use, Blomstrom said. Pricing for the CCD-based Diamond has not yet been determined, but will likely range from $200,000 to $250,000, he said.
While it moves forward with its digital development efforts, Instrumentarium isn't neglecting its traditional film-screen business. Along with Diamond, the firm will debut two other systems at the NCBC conference. Alpha RT is being reintroduced to the U.S. market after a hiatus of several years. The system was taken off the market in favor of the company's Alpha IQ product, but is being brought back as an entry-level system.
Another system, Performa, is designed to serve as a high-productivity workhorse unit. Performa includes the firm's EPS Compression System, which allows for compression below the breast, enhancing patient comfort. Performa also features Diamond's new x-ray tube, which offers improved gaussian technology that helps maintain focal-spot tolerance. Performa costs $110,000, while Alpha RT lists at $88,700.
By Erik L. RidleyAuntMinnie.com staff writer
April 7, 2000
(This is the fourth in a series of articles on digital mammography technology. Click on the headlines below to view previous articles.)
Siemens seeks to cut full-field digital mammo costs. March 24, 2000Fischer eyes clearance for digital mammography. February 22, 2000
Digital mammography to face tough implementation hurdles. February 7, 2000
Let AuntMinnie.com know what you think about this story.