Lymphoscintigraphy
Chemistry and Pharmacology
Tc-Antimony trisulfide colloid was previously the agent of choice for nuclear lymphoscintigraphy, but this agent is no longer commercially available. Other radiocolloids are therefore used and include Tc-sulfur micro- or nanocolloid (which is Tc-Sulfur colloid filtered through a millipore filter to eliminate all particles larger than 0.22 microns in diameter) or Tc-human serum albumin (HSA). The permeability of lymphatics to colloidal particles is maximal for particles less than 50 nm. Larger particles remain mostly at the injection site.
An inherent limitation of lymphoscintigraphy is its poor visualization of the deep lymphatic system. Web space injections highlight the superficial system only. Deep lymphatic channels originating posterior to the malleoli and coursing to the popliteal nodes and then along the superficial femoral vein are usually not identified.
Following the intradermal injection of Tc-HSA or Tc-nanocolloid (1 mCi [37 MBq] each foot), activity normally appears in the lymphatic bundle along the anteromedial aspect of the leg. Several channels may be identified in the calf, but only one vessel is seen in the thigh. Activity can appear within the pelvic nodes within 10 minutes due to the small size of the particles. Muscular exercise (walking) is used to enhance lymphatic flow.
Lymphedema:
Lymphedema results from the progressive accumulation of protein rich fluid in the interstitial spaces secondary to an anatomic or functional obstruction of the lymphatic system.
Primary lymphedema is the result of congenital aplastic or hypoplastic lymphatics. If familial it is referred to as Noone-Milroy's disease, but this is a rare disorder. Primary lymphedema is classified by the age of onset- congenital, or praecox (prior to) and tarda (over the age of 35, respectively). The disorder is slowly progressive, tends to affect females (4:1 predominance) with a peak incidence at puberty, and a lower limb is involved in the majority of cases.
In patients with congenital lymphatic aplasia, scintigraphy typically demonstrates little to no removal of tracer from the injection site, no lymphatic channels, and no regional nodal activity. A decreased number and or caliber of lymphatic vessels is seen in patients with hypoplasia.
Secondary lymphedema occurs when there has been some type of insult to the normal
lymphatic channels (surgery, trauma, neoplasm, or inflammation). Worldwide, the most
common cause is filariasis due to Wuchereria bancrofti infection.
Microangiolymphatic surgery is usually more successful in secondary lymphedema than in
primary lymphedema. Scintigraphy typically demonstrates delayed transport of tracer from
the injection site with dermal back flow, large collateral channels, and fewer visualized
lymph nodes. Cross-over of activity from the contra-lateral normal side to pelvic nodes on
the affected may also be seen and care should be taken to ensure that this finding is not
misinterpreted as normal.
Sentinel Node Detection:
Clinical: Up to 20% of patients that present with melanoma and no clinical evidence of metastases harbor occult lymph node metastases. Patients at greatest risk for lymph node metastases are those with a lesion depth of more than 1 mm Breslow thickness. The presence of regional lymph node metastases is a very important predictor of patient 5 year survival [3]. Prophylactic elective lymph node dissection can result in a 50% 5 year survival rate when micrometastases are found (as compared to a 25% survival rate when patients subsequently present with enlarged lymph nodes) [3]. Unfortunately, morbidity from lymph node dissection is high. Lymphoscintigraphy with lymph node mapping can be used to better delineate the lymphatic drainage from the primary lesion. The procedure is based on the hypothesis that melanoma metastasizes to regional lymph nodes via a defined connection of dermal lymphatics which can be followed to the first or "sentinel" lymph nodes in the regional basin. The absence of tumor in the sentinal node is felt to be strongly indicative of the absence of metastases to other nodes in the regional basin. Thus, the patient could be spared extensive node dissection surgery if the sentinel nodes are negative. If the sentinel nodes are positive for tumor, then the patient would undergo a complete lymph node dissection [2].
Procedure: Tc-nanocolloid (1 mCi) is injected intradermally about the lesion or surgical site. Dynamic 60 second images are obtained for 30 to 45 minutes to demonstrate lymphatic flow and regional drainage. Lateral or oblique static images are also acquired to better depict the nodal findings. Delayed static images at 1, 2, and 4 hours may be required if the nodes are not well depicted on early images. Activity from the injection site may occasionally mask the regional nodes if they are located very near to each other. Shielding may be required. The sentinal node is usually evident as the primary lymphatic channel will drain to this node. More than one primary lymphatic channel may be identified and therefore, patients can have more than one sentinal node. Another benefit of lymphoscinitgraphy is that unanticipated patterns of lymphatic drainage can be identified and aid in directing surgical node dissection. Between 7.5-15% of melanoma patients can have dual drainage patterns [2]. For the procedure, the surgeon utilizes a hand-held gamma probe to identify radioactive nodes. Blue dye is also generally injected intradermally just prior to the procedure [3]. The count rate is higher in sentinal nodes and the first node in the line is usually the most radioactive [1]. Generally a count rate at least 3 times that of background is considered positive [3]. Blue staining of the node helps to confirm that the node is the sentinel node [3].
Outcome: Lymphatic mapping with sentinel node dissection has been demonstrated to be equivalent to elective lymph node dissection [2]. The procedure can provide prognostic information based upon the presence or absence of regional lymph node metastases [3]. Although the procedure reduces the morbidity associated with elective lymph node dissection, lymphoscintigraphy and sentinel node dissection has not been show to result in overall disease-free survival [2]. The rate of regional lymph node recurrences is also similar between the two procedures [2].
REFERENCES:
(1) J Nucl Med 1997; Kapteijn BA, et al. Validation of gamma probe detection of the
sentinal node in melanoma. 38: 362-366
(2) Ann Surg Oncol 1999; Essner R, et al. Efficacy of lymphatic mapping, sentinel node lymphadenectomy, and selective complete lymph node dissection as a therapeutic procedure for early-stage melanoma. 6: 442-449
(3) Cancer 2001; Statius Muller MG, et al. The sentinel lymph node status is an important factor for predicting clinical outcome in patients with stage I or II cutaneous melanoma. 91: 2401-08
Breast Cancer:
Axillary node status is required to properly stage breast cancer and the presence or absence of axillary node metastases carries important prognostic information [3]. Conventional axillary node dissection surgery suffers from sampling error and can be complicated by significant patient morbidity (particularly lymphedema in the ipsilateral arm) [3]. The primary route of lymphatic drainage from a breast lesion will initially be to a single node (the "sentinal node"). The absence of tumor in the sentinal node (SLN) is felt to be strongly indicative of the absence of metastatic disease to other nodes in the regional basin. Thus, patients could be spared extensive node dissection surgery if the "sentinel node" could be identified, examined, and found to be free of tumor. Further axillary dissection could be performed only in patients with positive findings for malignancy in the sentinal node.
Lymphoscintigraphy has been used in the detection of the sentinal node in patients with breast cancer. The axillary sentinal node can be properly identified in 90 to 100% of patients with use of an intraoperative gamma ray detecting probe and blue dye [2,10,11]. The technical success rate for the exam has been reported to be between 66% to 100%. The exam has a reported sensitivity of 83-100%, specificity of 100%, positive predictive value of 100%, negative predictive value of 92-100%, and accuracy of 95-100% [4]. Lymphoscintigraphy can also provide information regarding primary drainage to sites other than the axilla such as the inaccessible internal mammary chain (approximately 6-10% of cases [3,10]). Up to 46% of tumors with drainage to internal mammary nodes can be located within the outer part of the breast [10]. Although the presence of "skip" metastases (axillary node metastases at level II or elsewhere with no evidence of metastases at level I) has been reported to be 10-12%, these "skip" lesions likely represent sentinal nodes outside level I. These nodes can be readily identified by lymphoscintigraphy [4].
Indications: Patients who are being considered for axillary node dissection and who do not have clinically involved nodes by exam or high tumor grade (T3 or T4) are candidates for lymphoscinitgraphy. Patients with nodes that are felt to be involved by tumor on clinical exam are not candidates for lymphscinitgraphy as lymphatic flow to a node that is replaced by tumor may be obstructed and the node may not be identified (ie: incorrect localization of the sentinel node) [12,14]. Surgical biopsy can disrupt lymphatic pathways and may affect exam results [4], although other authors have found no difference in sentinel node localization following prior surgical biopsy [10,11,15].
Agent/Procedure: Tc-sulfur colloid (Tc-SC) is the agent most commonly used for sentinel node localization. Unfortunately, there is no standardized technique for sentinel node localization. Wide variations exist regarding particle size, timing of injection, volume and placement of the injection, use of pre-operative imaging, and operative techniques [11].
Particle size: In some centers, the Tc-SC particle size is reduced to aid in lymphatic uptake of the tracer. A reduction in particle size is accomplished by use of shortened boiling times during preparation or by using Tc-pertechnetate of longer ingrowth, and filtering the agent through a 200 nm millipore filter [3]. Not all centers use filtered colloid as they feel there is improved/prolonged retention of the agent in the sentinal nodes when the colloid is not filtered [4,9,11].
pH: Some centers adjust the pH of the injected agent to neutral with sodium bicarbonate [5].
Injection site: Peri-tumoral, intradermal, or intra-lesional injection can be performed. Injection into a biopsy cavity, seroma, or hematoma may result in poor or no migration [3] and is not recommended. Prior to surgical biopsy, 1% isosulfan blue dye is also commonly injected around the margins of the tumor (intraparenchymal) in order to aid in SLN localization. The combination of isotope and blue dye have a complementary effect in sentinel node localization [10-12,14].
1- Intraparenchymal/peritumoral injection: It would seem logical that a peri-tumoral injection of the tracer would most accurately reflect the lymphatic drainage of the lesion [11]. Approximately 0.5 - 1.0 mCi of the agent in a total volume of 3 to 7 ml is injected around the periphery of the tumor [3]. If the lesion is not palpable, a single injection can be performed through a localizing wire introducing needle [5]. Due to the several injections made with this technique, there may be a "blooming" of the tracer activity which can obscure the axilla or internal mammary node area on imaging [11]. Some authors also report a decrease in SLN identification with this technique compared to an intradermal injection [11].
2- Intradermal injection: Intradermal injections are performed by some centers using a smaller dose and volume (0.1 mCi in 0.5 mL) [9]. The lower injected activity is associated with less "blooming" and the technique is easier to perform than peri-tumoral injections [11,14]. Tracer migration is also faster using a subdermal injection [16]. Localization of the SLN with this technique is excellent [10,11,14] and a dermal injection may actually be superior to a peri-tumoral injection for SLN identification [11,15]. Intradermal injections may have a lower detection of intramammary nodes [16].
3- A single intratumoral injection is an alternative method and is also a valid way to identify the lymphatic drainage from the lesion [6].
Imaging: Imaging should begin within a few minutes of injection, however, dynamic imaging is not required as it is for sentinel node localization in melanoma [16]. The patient should be placed in a semirecumbent position with the ipsilateral shoulder elevated to about 45 degrees by a triangular foam wedge and the arm raised over the head. This position helps to separate injection activity from axillary node activity [7]. An anterior image is still useful to obtain for evaluation of activity in the internal mammary nodes [7]. The mean time to visualization of the sentinel node is about 20 minutes [3], but imaging can take up to 2 hours. Once identified, the sentinel node should be further localized and marked on the skin using a hand-held probe with the patient in the same position that will be used in surgery. If imaging is delayed, multiple nodes may be seen and localization of the sentinel node may not be possible. Nodes located close to the injection site may sometimes be obscured by scatter radiation. Adjusting the energy window to sample only the high side of the technetium photopeak can assist in identifying these nodes (ie: use 15% energy window centered at 151 keV) [3]. An outline of the patients silhouette traced with a Tc-99m point source (< 0.5 mCi) or with a sheet source behind the patient aids in image interpretation [3]. If a sentinel node is not identified on scintigraphic imaging, a sentinel node can still often times be localized at surgery- either with a handheld probe or with blue dye [10]. However, in patients that fail to have a sentinel node identified during imaging, there is a higher rate of failure to identify a SLN at surgery (19% of patients with non-visualized SLN on imaging had no SLN identified at surgery, as compared to only 1% of patients with visualized SLN at imaging) [10]. Patients over the age of 50-60 years are also more likely to have failure to localize the sentinel node [10,11].
A two day protocol has also been evaluated using unfiltered Tc-sulfurcolloid with results similar to the one day exam (sensitivity for sentinel node localization was 97%) [9]. This protocol permits greater flexibility for patients and operating room time is not wasted waiting for patients to arrive from lymphoscintigraphy [9]. A higher dose of Tc-sulfurcolloid is used (0.5 mCi or 18.5 MBq) to allow for radioactive decay of the agent.
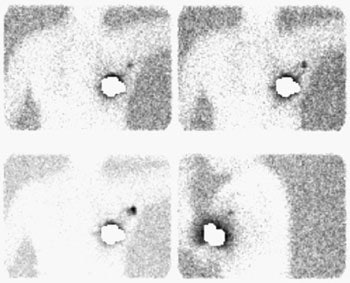
Sentinel lymph node imaging: The lymphoscintigraphy exam below was performed using a peri-tumoral tracer injection. Images were performed at 5, 10, and 15 minutes after injection in a LAO projection with the left arm behind the patient's head. A lead shield was placed over the breast. The exam demonstrated rapid localization of the primary lymphatic drainage to the axilla. Following the procedure the patient went to the operating room for gamma probe localization of the sentinel node. |
|
At surgery, a hand-held gamma probe is used to localize the sentinel node. The count rate in the sentinel node should be at least 3 to 5 times axillary background [5,9,15]. Often, surgeons will also pre-operatively (5 to 10 minutes before axillary dissection) inject 1% isosulfan blue dye around the tumor site to aid in the visual identification of the sentinel node and lymphatic drainage. Blue due localization can identify a small percentage of sentinel nodes (3.5-11%) not found by isotope localization [9,15]. Gamma probe localization without lymphscinitgraphy imaging may be adequate for sentinal node localization [4,10]. Failure to identify the sentinel node is an indication for standard axillary dissection [3] as lack of identification of this node does not exclude the presence of axillary metastases [10]..
Dosimetry: The dose to the breast tissue is estimated to be approximately 45 rads per mCi of Tc-99m-sulfer colloid (1.2 cGy/MBq) assuming a distribution of the injected dose in 6 cc of tissue [3]. The estimate can vary by an order of magnitude depending on the volume of distribution and washout rate [3]. Resection of the breast tumor during surgery will help to decrease the radiation burden [3]. The surgeon receives a hand dose of about 9.4 +/- 3.6 mrem per case [3]. It is important to establish procedures for handling/processing specimens, monitoring personnel, and monitoring work areas for radiation for lymphoscintigraphic localizations [3].
Sentinel node localization is a team effort that requires close coordination between the surgical team, radiology and nuclear medicine. The surgeon and nuclear medicine physician should have a clear understanding regarding the meaning of skin marks, patient position, and sources of artifacts [3]. The use of both a blue dye and radioscinitgraphy will give the benefit of visual cues and count-rate data for the surgeon [3,4]. A learning curve exists, and success improves with experience [4]. Each institution must set up a program with strict technical guidelines to confirm the accuracy of the examination [12]. The American Society of Breast Surgeons recommends that surgeons anticipating the use of sentinel node biopsy as their sole means of staging the axilla should first perform 30 validation cases with accurate identification of the sentinel node 85% of the time and a false-negative rate less than or equal to 5% [13].
REFERENCES:
(1) J Nucl Med 1997; Pijpers R, et al. Impact of lymphoscintigraphy
on sentinal node identification with technetium-99m-colloidal albumin in breast cancer.
38: 366-68
(2) J Nucl Med 1998; De Cicco C, et al. Lymphoscintigraphy and radioguided biopsy of the sentinel axillary node in breast cancer. 39: 2080-2084
(3) Semin Nucl Med 1999; Glass EC, et al. Sentinel node localization in breast cancer. 29 (1): 57-68
(4) Radiology 1999; Liberman L, et al. Sentinel lymph node biopsy after percutaneous diagnosis of nonpalpable breast cancer. 211: 835-844
(5) Radiology 1999; Eary JF, et al. Sentinel lymph node mapping for breast cancer: Analysis in a diverse patient group. 213: 526-529
(6) J Nucl Med 2000; Valdes-Olmos RA, et al. Evaluation of mammary lymphoscintigraphy by a single intratumoral injection for sentinel node identification. 41: 1500-1506
(7) J Nucl Med 2000; Haigh PI, et al. Factors affecting sentinel node localization during preoperative breast lymphscintigraphy. 41: 1682-1688
(8) AJR 2001; Tuthill LL, et al. Biopsy of sentinel lymph nodes guided by lymphoscintigraphic mapping in patients with breast cancer. 176: 407-411
(9) J Nucl Med 2001; Yeung HW, et al. Lymphoscintigraphy and sentinel onde localization in breast cancer patients: A comparison between 1-day and 2-day protocol. 42: 420-423
(10) Radiology 2001; Birdwell RL, et al. Breast cancer. Variables affecting sentinel lymph node visualization at preoperative lymphscintigraphy. 220: 47-53
(11) Ann Surg Oncol 1999; Linehan DC, et al. Intradermal radiocolloid and intraparenchymal blue dye injection optimized sentinel node identification in breast cancer patients. 6: 450-454
(12) Ann Surg Oncol 2001; Grube BJ, Giuliano AE. Modification of the sentinel node technique: It was a hit in New York, but will it play in Poughkeepsie? 8: 3-6 (No abstract available)
(13) Ann Surg Oncol 2001; Zervos EE, et al. Localizing the sentinel node outside of the specialty center: Success of a lymphatic mapping course in disseminating new technology. 8: 7-12
(14) Ann Surg Oncol 2001; Boolbol SK, et al. Intradermal isotope injection: A highly accurate method of lymphatic mapping in breast carcinoma. 8: 20-24
(15) Ann Surg Oncol 2001; Cody HS, et al. Complementarity of blue dye and isotope sentinel node localization for breast cancer: Univariate and multivariate analysis of 966 procedures. 8: 13-19
(16) Society of Nuclear Medicine Annual Meeting Handout Book 2001; Keshtgar MRS, et al. The sentinel node in breast carcinoma- present controversies. 80-92 (No abstract available)