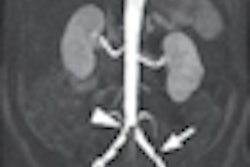
Bayer HealthCare Pharmaceuticals has received U.S. Food and Drug Administration (FDA) clearance for Gadavist (gadobutrol), a gadolinium-based contrast agent for contrast-enhanced MRI of the central nervous system.
Gadavist is indicated for intravenous use in adults and children (2 years of age and older) to detect and visualize disrupted blood-brain barrier and/or abnormal vascularity of the central nervous system, Bayer said. The product is known as Gadovist outside the U.S.
At 1 mmol/mL, the agent is formulated at a higher concentration than certain other gadolinium-based contrast agents, according to the firm. This results in half the volume of administration and a more compact contrast bolus, Bayer said.