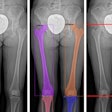
With the use of quantitative CT (QCT), researchers from the University of Minnesota determined that gynecologic cancer patients lost as much as one-fourth of their bone mineral density after undergoing chemotherapy and radiation therapy.
Radiation therapy and/or chemotherapy can successfully cure gynecologic cancers, but the women who received these treatments may be more susceptible to fractures from associated bone loss. To investigate these effects, physicians at the University of Minnesota School of Medicine in Minneapolis analyzed data from treatment-related CT scans to detect and measure abnormal changes in bone density.
Radiologists, radiation oncologists, and medical physicists at the university used quantitative CT to retrospectively investigate the impact of chemotherapy and radiation therapy on the volumetric bone mineral density of 40 women. These ovarian, endometrial, and cervical cancer patients had undergone at least three pre- and post-treatment CT scans between 2005 and 2009 (American Journal of Obstetrics and Gynecology, October 2010, Vol. 203:4, pp. 353-357).
Images were acquired from the hospital's PACS, downloaded to a computer, and analyzed with quantitative CT software to determine bone density (Mindways Software, Austin, TX), according to lead author Susanta Hui, PhD, of the department of therapeutic radiology, and colleagues.
The patient cohort included 12 women with ovarian cancer (median age, 61 years), who exclusively received chemotherapy. Ten women (median age, 56) with endometrial cancer received pelvic external-beam radiation therapy (EBRT), with a median dose of 45-50 cGy in 25 to 28 fractions, and an additional radiation dose of 5-18 Gy at the vaginal surface using high-dose-rate brachytherapy.
An additional 18 women with cervical cancer (median age, 47) received radiation-sensitizing chemotherapy once a week as they received extended-field EBRT treatments. EBRT field and radiation doses were individualized according to tumor size, lymph node involvement, and the performance status of the patient. The median radiation dose for this group was 45-50 cGy delivered in 25 to 28 fractions, as well as 20-40 Gy from one to two implants of low-dose-rate brachytherapy.
The research team compared the volumetric bone mineral density at baseline prior to any treatment and at two time points after completion of the treatment. The bone mineral density level at the trabecular regions of the spine and the femoral neck region were measured on each scan, and differences were computed. The mean interval from the baseline CT scan to the first and second follow-up scan was 7.5 months and 12.5 months, respectively.
Cervical cancer patients, who received both chemotherapy and radiation therapy, had the highest volumetric bone mineral density reduction, at 23.9%. In comparison, those who received chemotherapy only (ovarian cancer patients) lost 15.2%, and those who underwent high-dose-rate treatment (endometrial cancer patients) lost 11%.
Bone loss varied by anatomic region and the treatment received. The combined chemotherapy/radiation therapy treatment had the greatest effect on bone loss at the L5 trabecular region of the spine, whereas radiation therapy treatment alone had the greatest effect at the femoral neck region.
The researchers identified that postmenopausal ovarian cancer patients were at high risk for bone loss, with major loss occurring within eight months of treatment. Although radiation was shown to cause rapid bone loss within six months, the total reduction in bone loss occurred in patients who underwent chemotherapy, with or without additional radiation therapy.
Use of quantitative CT is more advantageous than ordering a dual-energy x-ray absorptiometry (DEXA) scan, according to the authors. Not only does this eliminate the cost of a DEXA exam, DEXA scans do not offer the flexibility to measure pelvic areas that may have the greatest mineral loss, and they cannot provide precise localization of cortical or medullary calcium loss, the group wrote.
Long-term follow-up studies should be conducted to determine if anticancer therapies affect bone loss for longer periods of time, Hui and colleagues noted. Such studies would also help determine if there is a natural recovery process from skeletal damage for different treatment modalities, they wrote.
By Cynthia E. Keen
AuntMinnie.com staff writer
November 26, 2010
Related Reading
Automated QCT offers hope for passive osteoporosis screening, December 28, 2009
Secondary causes of bone loss may be missed in breast cancer, August 20, 2009
MDCT holds promise for advanced osteoporosis scan, April 6, 2009
Road to RSNA, Women's Imaging, Mindways Software, November 9, 2006
Copyright © 2010 AuntMinnie.com