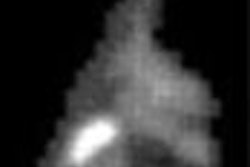
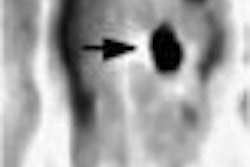
Despite previous rumblings that Medicare payment for imaging breast cancer with PET cancer was a no-go, an advisory committee to the Centers for Medicare and Medicaid Services (CMS, formerly known as HCFA) voted Tuesday to accept recurrent breast cancer as an indication for the use of PET.
Less than two weeks ago, ReutersHealth reported that the CMS administrators were set to speak out against the use of the modality for breast cancer imaging. Their opinion was based on an independent assessment conducted by the Blue Cross Blue Shield (BCBS) Technology Evaluation Center. Financed by CMS, the study determined that the risk of a false-negative result is around 12% for patients with a 50% chance of having a malignancy, and about 29% for patients with a 75% chance of malignancy.
But at the final hearing in Baltimore, PET proponents talked up the value of the modality for re-staging patients with breast cancer. It was apparently enough to convince the committee to approve guidelines for Medicare reimbursement of PET for recurrent breast cancer.
Dr. Peter Conti, chairman of the Society of Nuclear Medicine scientific program committee, praised the committee’s decision, adding that PET imaging can be invaluable for patients with advanced breast cancer, particularly those who are candidates for additional treatment.
Related Reading
HHS officials change name, structure of Medicare agency, June 15, 2001
SNM says HCFA understates PET's value in breast imaging, June 8, 2001
Medicare coverage of PET imaging for breast cancer to be challenged, June 8, 2001
Copyright © 2001 AuntMinnie.com