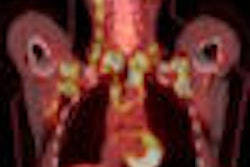
The U.S. Food and Drug Administration (FDA) has accepted GE Healthcare's new drug application for DaTscan (ioflupane iodine-123 injection) for priority review.
DaTscan is the Chalfont St. Giles, U.K.-based company's radiopharmaceutical intended for detecting loss of functional nigrostriatal dopaminergic neurons in SPECT imaging in patients presenting with symptoms or signs suggestive of dopaminergic neurodegeneration.
The FDA's priority review designation is intended for drugs that offer major advances in treatment or provide treatment where no adequate therapy exists.
DaTscan has been available since 2000 in Europe, where it is indicated for detecting loss of functional dopaminergic neuron terminals in the striatum in patients with clinically uncertain Parkinsonian syndromes, to help differentiate essential tremor from Parkinsonian syndromes related to idiopathic Parkinson's disease (PD), multiple system atrophy (MSA), and progressive supranuclear palsy (PSP).
DaTscan is unable to discriminate between PD, MSA, and PSP, the company said.
Related Reading
GE partners with LightLab, May 29, 2009
GE wins CZT gamma camera installs, May 28, 2009
GE unveils partnerships at Heart Rhythm 2009, May 18, 2009
GE to highlight technologies at Heart Rhythm 2009, May 15, 2009
GE, Varian Inc. launch 7-tesla MRI unit, May 13, 2009
Copyright © 2009 AuntMinnie.com