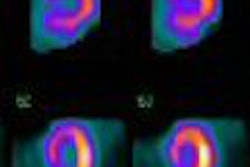
Molecular Insight Pharmaceuticals has begun a phase I clinical trial of its Azedra (Ultratrace iobenguane I-131, formerly known as Ultratrace MIBG) molecular imaging pharmaceutical for the treatment of neuroendocrine tumors such as carcinoid, pheochromocytoma, and neuroblastoma.
The dosimetry trial is designed to evaluate the safety, tolerability, and distribution of Azedra in adult patients with one of two forms of neuroendocrine cancer -- either carcinoid or pheochromocytoma, according to the Cambridge, MA-based firm. The study is being conducted at Duke University in Durham, NC.
The company reported that Azedra, which was recently granted orphan drug status by the U.S. Food and Drug Administration, was also granted fast track status by the FDA.
By AuntMinnie.com staff writers
June 22, 2006
Related Reading
Molecular Insight begins phase II clinical trial, March 27, 2006
MDS Nordion, Molecular Insight Pharmaceutical partner, February 16, 2006
Molecular Insight fills executive ranks, July 21, 2005
Molecular Insight hires new vice presidents, June 1, 2005
Molecular Insight raises $28 million, June 1, 2005
Copyright © 2006 AuntMinnie.com