Since PET imaging was introduced in the 1990s, its advocates have slowly but surely established a place for the technology, so much so that PET is now considered by many to be the best modality for functional oncology imaging.
PET's rising star has helped the modality eclipse SPECT, which has long been used for oncology applications but lacks PET's image quality. Sales of SPECT gamma cameras have plateaued for the last several years, while PET equipment sales have grown at a much more rapid clip, according to market research firm IMV Medical Information Division of Des Plaines, IL.
Even so, dissenting voices continue to offer critical perspectives on PET, particularly its cost and accessibility. There were nearly 13,000 gamma cameras installed in the U.S. in 2003, compared with about 755 fixed and mobile PET and PET/CT systems in 2003, according to IMV. SPECT isotopes also tend to have longer half-lives than fluorodeoxyglucose (FDG), the workhorse PET radiopharmaceutical.
Biotechnology company Cell>Point, with dual headquarters in Houston and Englewood, CO, hopes to capitalize on the large worldwide installed base of SPECT cameras by developing radiopharmaceuticals in new combinations. Their hope is to make oncology imaging as versatile and accurate on a SPECT system as it is on a PET camera.
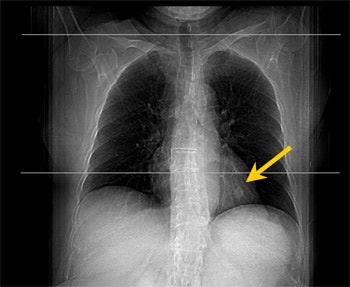 |
| CT image of suspicious 1.4-cm pulmonary nodule in left lower lobe. |
The company's primary platform, EC Technology (ethylenedicysteine drug conjugate technology), is based on ethylenedicysteine, a stable water-soluble chemical coupler. EC Technology allows Cell>Point to create radioisotope combinations that may not have been connected before, such as the radiotracer technetium paired with deoxyglucose, the other half of FDG.
In addition to developing a new class of molecular imaging agents, Cell>Point is using EC Technology to widen the application of particular cancer drugs by allowing them to be used for radioimmunotherapy, and to create new intracellular radiotherapeutic agents.
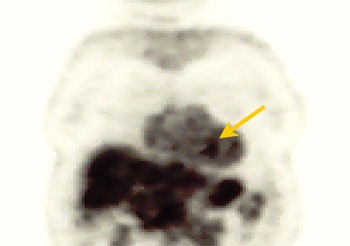 |
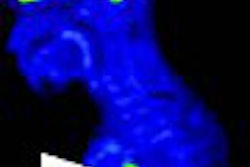
| PET study from the same patient indicates very faint uptake activity that may be indicative of malignant process (SUV of 2.1). |
In partnership with multimodality vendor Philips Medical Systems of Andover, MA, Cell>Point plans to enter phase II/III clinical trials in June for the company's first product, technetium-99m-EC-deoxyglucose (99mTc-EC-DG), a metabolic imaging agent that will enable the diagnosis of hypercellular activity in cancer cells. The company expects these trials to be completed by May 2006.
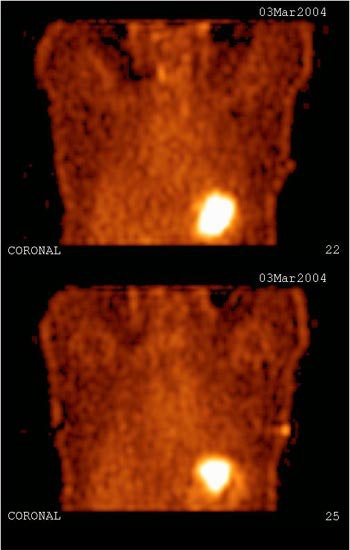 |
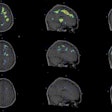
| Study of the same patient with 25.4 mCi of Tc-99m-labeled EC-DG shows no focal areas of abnormality. Activity is noted in the stomach as well as the thyroid, indicating the presence of free pertechnetate. Images courtesy of Cell>Point. |
Established in 2001 by brothers Terry and Greg Colip, a scientist and lawyer, respectively, and molecular biologist Jerry Bryant, Cell>Point was born when the founding members acquired what became EC Technology. The technology had been developed at the University of Texas M. D. Anderson Cancer Center, also in Houston, by another trio, David Yang, Ph.D.; Dr. Tony Yu; and Dr. Edmund Kim.
Yang, Yu, and Kim had begun research on alternatives to SPECT in the mid-1990s, manipulating ethylenedicysteine to make it an effective chelator. Bryant connected the researchers to the Colips, and the three founded Cell>Point for the purpose of acquiring technology developed at M. D. Anderson.
The company has acquired two more technologies since its inception, and a fourth is in the works. For each platform Cell>Point has obtained from M. D. Anderson, the company retains an exclusive worldwide patent and technology license. Cell>Point also has five-year sponsored research agreements for each platform with M. D. Anderson.
"(Our model is to) acquire the technology, support its development and the clinical validation of products out of the technology, and then hand the ball off to a licensee, a large radiopharmaceutical firm that already has a footprint in manufacturing, marketing, and distribution," Greg Colip said. "And since the ongoing product development happens in M. D. Anderson labs, we can rely on their inventor-scientists and staff rather than having to go out and secure independent lab space."
Cell>Point hires manufacturers with certified good manufacturing practices (cGMP) to batch-produce product kits, and engages clinical research organizations to monitor clinical trials. The company doesn't file its own new drug applications (NDAs) with the Food and Drug Administration for the products developed from the platform, but rather licenses them to large radiopharmaceutical or pharmaceutical companies. Cell>Point is in discussion with potential partners.
Since its products will be used with gamma cameras, Cell>Point predicts that the cost of a SPECT procedure performed with 99mTc-EC-DG will be less than half the cost of an 18F-FDG-PET scan, which should revitalize the use of SPECT and make oncology imaging more accessible for rural and community hospitals, and oncology clinics, according to Greg Colip.
"Diagnostic oncology work has typically been done with FDG-PET," he said. "But there are only about 1,000 PET cameras installed in the U.S., while there are more than 15,000 SPECT cameras installed across the country. The market opportunity is significant, and we're working to bring out a lower-cost molecular imaging agent for diagnostic and therapeutic procedures."
There could also be clinical benefits to its EC Technology versus PET scanning, according to the company. Clinical studies indicate that 99mTc-EC-DG is not taken up in inflammatory tissue, which suggests that 99mTc-EC-DG can differentiate between inflammation and tumor.
In addition to 99mTc-EC-DG for oncology and cardiology applications, Cell>Point has plans for other technetium-based agents:
- 99mTc-EC-LHRH for ovarian and endometrial imaging
- 99mTc-EC-annexin V for apoptosis targeting
- 99mTc-EC-metronidazole for hypoxia targeting
- 99mTc-EC-guanosin for prostate imaging
The company also plans to develop therapeutic agents using rhenium:
- 188Re-EC-DG, an intracellular agent that targets and treats solid tumors
- 188Re-EC-LHRH to treat ovarian and endometrial cancers
- 188Re-EC-guanosin to treat prostate cancer
Cell>Point hopes to launch 99mTc-EC-DG in 2006, have 11 products in clinical trials in 2007, and 12 products on the market by 2009.
EC Technology not only will take advantage of the SPECT installed base, it will offer doctors and patients less intrusive disease diagnoses and therapies, according to Terry Colip, managing partner and CFO.
"Cell>Point's technology should be more effective in diagnosing and treating many forms of cancer than other diagnostic and therapeutic technologies available, and should prove much more humane than chemotherapy," he said.
By Kate Madden Yee
AuntMinnie.com contributing writer
May 20, 2005
Related Reading
SPECT reveals sharply higher cardiac risk within metabolic syndrome, April 22, 2005
PEM development picks up pace, February 7, 2005
Copyright © 2005 AuntMinnie.com