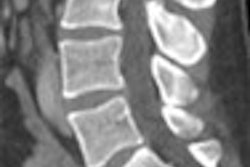
Image display and advanced visualization software developer Barco of Kortrijk, Belgium, has received 510(k) clearance from the U.S. Food and Drug Administration for its CardiaMetrix software.
CardiaMetrix features clinical applications for coronary artery vessel analysis, calcium scoring, 4D cardiac analysis, and left ventricular functional analysis, and is an option on Barco's Voxar 3D software suite for advanced visualization and analysis.
By AuntMinnie.com staff writers
June 14, 2006
Related Reading
Barco gets FDA 510(k) for Voxar 3D Enterprise, April 28, 2006
Barco adds surgical display, March 21 2006
New software, display introductions for Barco in Europe, March 6, 2006
Barco launches 2-MP color display, January 24, 2006
Barco, NeuroLogica sign development deal, December 13, 2005
Copyright © 2006 AuntMinnie.com