If you're a small company just getting started in the cutthroat medical imaging industry, it helps to have influential friends. That could be the motto of U.K. software developer Medicsight, which has lined up an impressive array of clinical partners as it begins to bring its line of computer-aided detection (CAD) products to market.
Medicsight was founded in 1999, but the company began drawing attention earlier this year when it announced its involvement in the International Early Lung Cancer Action Project (I-ELCAP), a large international study to research the use of CT for lung cancer screening. Medicsight has also landed a number of other high-profile luminary partners in other clinical segments it's targeting for its CAD software.
While CAD is best known for its growing role in mammography screening, Medicsight is focusing its development efforts on three main clinical areas outside the breast: colon, lungs, and heart. These segments are similar in that they represent areas where population-based screening programs could have widespread clinical benefits. Few such programs have been set up, however, due to the challenge involved in reviewing the massive amounts of data produced by CT screening studies.
"You'll never develop screening programs, particularly in the colon, because looking at these thousand slices you get from the colon or 500 or 600 you get from the lung is tedious and extremely time-consuming, and therefore expensive, and human error creeps in," said John Costello, medical director of the company. "You have to have software. If you don't have the software, you won't have the screening programs."
Medicsight's software has its roots in research conducted on pattern-matching algorithms at a U.K. firm called HTTP Technology. HTTP originally hoped to develop software for military defense applications, but later chose to refocus on the medical market for CAD software. It changed its named to Medicsight to reflect its new emphasis.
Medicsight at one point planned to open a chain of imaging centers in the U.S. and U.K. under the Lifesyne brand, but later abandoned that strategy to focus on CAD software. It still retains one Lifesyne center in London, which provides imaging services to patients while also serving as a test site for the company's software.
Going beyond the second reader
At first glance, Medicsight's software functions like many other CAD applications: It analyzes scanned image data and highlights suspicious areas on an image that appears side by side with the original scan on the Medicsight user interface. But Medicsight's first product line, its computer-assisted reader (CAR) software for the lung and colon, is designed to be used as the radiologist reviews the original image. This differs from CAD software products currently on the market that are employed after the original image has been read, as a sort of computer-based second reader. Medicsight hopes that its concurrent-reading technique will help radiologists save time when reviewing large numbers of images.
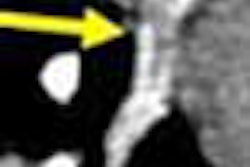
Another key point of differentiation, according to Medicsight, is the software's ability to analyze and measure the borders of suspicious lesions with extreme precision. Accurate boundary measurement is the key to effective CAD, Costello said, because one of the most effective ways of characterizing malignant from benign lesions is by measuring their rate of growth, due to the faster growth rates of cancerous tissue.
Medicsight believes that its software is so accurate that it can measure changes in nodule volume over time periods as short as six weeks. For example, a suspicious nodule might grow from 4 mm to 5 mm in diameter over a six-week span. While such a change might be unnoticeable to even the best-trained eye, it would represent a doubling of the nodule's volume, and thus could indicate the presence of a cancerous lesion, Costello said.
Clinical research partners
Medicsight believes that strong partnerships with clinical researchers are key to the company's success. In each of its three core areas of focus, the firm has sought to partner with clinicians who have access to large databases of scanned images that can be used to train and validate the software with histologically proven cases.
In lung imaging, Medicsight began working with the I-ELCAP group in late 2003 after meeting with lead researchers Dr. Claudia Henschke and Dr. David Yankelevitz. One study with the I-ELCAP group concentrates on the accuracy of the software's boundary-extraction algorithm in a cohort of live patients, Costello said.
Medicsight's work in colon imaging has been carried out in collaboration with Dr. Perry Pickhardt, the University of Wisconsin Medical School researcher who published a landmark study on virtual colonoscopy in December 2003, and Dr. Steve Halligan, an equally prominent VC researcher at St. Mark's Hospital in the U.K. As with the lung application, Medicsight is taking the results that its software has produced and is validating them against cases that have been proved by optical colonoscopy.
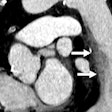
In the heart, Medicsight is concentrating on analyzing and measuring the growth rate of coronary calcium, which has been shown to be a strong indicator of future cardiac events. The firm hopes its software can address weaknesses in current methods of assessing coronary calcium, some of which lack reproducibility and overestimate plaque burden. The company's cardiac clinical partners include Centre Hospitalier Universitaire Vaudois at the University of Lausanne in Switzerland and Cornell University in New York City.
The company is rolling out its product line in stepwise fashion, according to David Sumner, international marketing director. The first offering in the colon and lung segments will be a CAR-based program, which includes several algorithms for filtering data. For the heart segment, Medicsight plans to offer HeartScreen, which provides calcium analysis and measurement tools rather than computer-assisted reading.
Farther down the road in the colon and lung segments will be CAD programs that include far more filters, with higher sensitivity and specificity. Medicsight hopes its implementation of CAD will be more ambitious than what other CAD firms currently have on the market. The company envisions a CAD program that scans image datasets and returns to radiologists only images that have suspicious areas that require review -- without requiring radiologists to read every image in the dataset.
Medicsight received Food and Drug Administration clearance for its Lung CAR software in August, and already has the CE mark for the product in Europe. FDA clearance for Colon CAR is expected in the third quarter of 2004. HeartScreen should be cleared sometime in the next two months.
Due to their increased complexity, Lung CAD and Colon CAD will have to go through the premarket approval (PMA) process in the U.S., Sumner said. He declined to provide an estimate on when the products would reach the market.
As the regulatory approvals roll in, Medicsight has begun efforts to raise its corporate profile. The company will exhibit for the first time at this week's European Society of Gastrointestinal and Abdominal Radiology (ESGAR) meeting in Rome, and will follow up with demonstrations at the International Symposium on Virtual Colonoscopy in October and the RSNA conference in December.
Medicsight's sales and marketing strategy is based on finding distribution partners for its software rather than selling it itself, according to Costello. The firm is in discussions with various potential partners, and hopes to have a deal cemented shortly, he said. Medicsight believes that a good strategic fit would be a multimodality vendor or a large PACS company that could incorporate the software into its offerings, Sumner said.
Ultimately, Medicsight would like to see its products become more than just second readers for radiologists -- they would become integral parts of the diagnosis and management of patients participating in populated-based screening programs for diseases that are the biggest killers in the developed world.
"Our vision is to take CAD software beyond detection so it's not just computer-aided detection, but a move toward computer-aided diagnosis -- diagnosis and patient management once diagnosed," Sumner said.
By Brian Casey
AuntMinnie.com staff writer
September 20, 2004
Related Reading
Medicsight to bring Lung CAR to market, August 16, 2004
Medicsight to restate 2001, 2002 results, January 20, 2004
Medicsight gets CE Mark, financing, October 8, 2003
Lifesyne CT screening center opens in London, May 14, 2003
Medicsight continues U.K. plans, eyes U.S. imaging center market, April 16, 2003
Copyright © 2004 AuntMinnie.com