Not all benign findings on screening mammography are equal. A Spanish team identified two characteristics of benign lesions that signal an increased risk of future malignancy in new research presented on October 3 at the 2020 European Breast Cancer Conference.
Women with benign breast disease (BBD) found after their first mammogram or with breast tissue with a proliferative presentation had a much higher risk of developing breast cancer than their peers. The findings could one day be used to better personalize screening mammography, according to presenter Marta Román, PhD, an epidemiology researcher at Hospital del Mar Medical Research Institute in Barcelona, Spain.
"Including information from BBD, in addition to other factors, to develop risk-based screening approaches can help with the prediction of whether a woman would develop breast cancer in a defined period," stated Román in a press release.
The retrospective study included data from 629,087 women who collectively underwent more than 2.3 million mammograms between 1995 and 2015. The researchers followed the patients for an average of almost eight years in order to see whether an association existed between characteristics of benign breast disease and breast cancer occurrence.
Out of roughly 629,000 women, clinicians diagnosed 9,184 incidences of benign breast disease and 9,431 breast cancers. The researchers noted there was a strong association between any type of BBD diagnosis and breast cancer risk, but that risk varied based on screening type and whether or not the disease was proliferative.
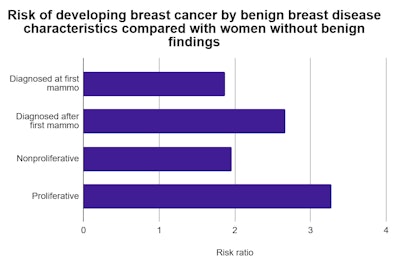
After adjusting for age at diagnosis and screening year, women with benign breast disease diagnosed at their first mammogram had 1.87-times the risk of developing breast cancer than women without a BBD diagnosis. But that risk ratio increased to 2.67 for women with a BBD diagnosed after their first mammogram.
The results were similarly stratified depending on whether the findings were proliferative, a signal of increased growth of certain cell types. Women with proliferative BBD had 3.28-times the breast cancer risk of women without benign findings. In comparison, women with nonproliferative BBD had a risk ratio of 1.96.
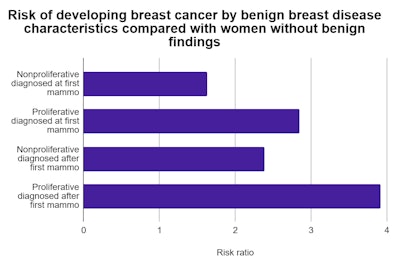
These findings were compounded for women diagnosed with proliferative benign breast disease after their first screening mammogram. These women had almost four times the breast cancer risk of women without benign findings. Meanwhile, women diagnosed with nonproliferative BBD at their first mammogram had a much lower risk ratio of 1.63.
Román and her colleagues hope to use the study findings to develop personalized mammography schedules based on a more comprehensive view of patient breast cancer risk. The team is currently participating in a six-country randomized clinical trial to test the effectiveness of risk-based breast screening.
"Personalized risk-based screening going beyond the current 'one size fits all' recommendation may increase the effectiveness of breast cancer screening," stated Román.