Year in Review, page 2
No. 4: Supreme Court upholds healthcare reform legislation
In one of the more surprising developments of the year, the U.S. Supreme Court in June upheld by a 5-4 vote President Barack Obama's healthcare reform plan -- including the so-called individual mandate, which requires all Americans to obtain healthcare insurance.
The court's decision, as well as Obama's re-election in November, dashed any hopes that the 2010 Patient Protection and Affordable Care Act (PPACA) would be overturned and that the issue of healthcare reform would just go away, according to Dr. Frank Lexa, from the Wharton School of the University of Pennsylvania.
 In June, the U.S. Supreme Court upheld PPACA.
In June, the U.S. Supreme Court upheld PPACA."The legislation can't be ignored anymore," Lexa said. "And radiologists need to be politically involved, so that whatever system develops [as PPACA is implemented], we'll have a central role in diagnosing and preventing disease."
Under PPACA, physicians and hospitals are going to be asked to generate savings and efficiencies in healthcare. In addition, the U.S. Centers for Medicare and Medicaid Services (CMS) will continue to take money from medical specialties and redistribute it to primary care to accommodate the influx of people who will now have healthcare coverage, according to Cynthia Moran, assistant executive director of the American College of Radiology (ACR).
"[At the ACR, we will work] to encourage CMS to promulgate policies that are based on evidence and facts and are supported by public scrutiny of their data and processes," Moran said. "We understand their political goals, but we want their actions to be based on rational judgment rather than possible prejudices."
The legislation is essentially payor reform, according to W. Kenneth Davis Jr., an attorney with Katten Muchin Rosenman in Chicago.
"The payor landscape is about to shift rather dramatically, and radiologists are going to have to become less focused on day-to-day practice changes and more aware of where reimbursement will be coming from and with whom they should ally," he told AuntMinnie.com.
By Kate Madden Yee
No. 5: Radiology gets seat at meaningful use table in stage 2
As hospitals and primary care providers enthusiastically embraced the U.S. government's "meaningful use" program to spur healthcare IT adoption, radiology could be forgiven if it felt like it hadn't been invited to the party. Although radiology was the first medical specialty to implement digital technology, it seemed to be an afterthought to the federal bureaucrats administering the program. That began to change in 2012.
A sign of the changing times was the announcement in early 2012 by the U.S. Centers for Medicare and Medicaid Services (CMS) that medical imaging would be added to stage 2 rules for implementing meaningful use. The announcement was widely seen as a victory for radiology and those who had worked hard to include the specialty in the meaningful use program.
The final stage 2 rule includes imaging as one of six menu objectives (of which three must be met). At least 10% of diagnostic imaging tests ordered by an eligible provider for patients admitted to an inpatient or emergency department during the electronic health record (EHR) reporting period will need to be accessible through certified EHR technology.
 Radiology finally made it to the meaningful use table in 2012.
Radiology finally made it to the meaningful use table in 2012.Although the stage 1 and 2 rules reflect baby steps instead of giant leaps, they will help spur the adoption of healthcare IT by referring physicians, which should lead to productivity improvements. They are also making it easier for radiologists to attest to meaningful use and be eligible for as much as $44,000 in payment per radiologist (if applied for by October 1, 2012), to say nothing of helping them avoid fines that begin in 2015 for not meeting the objectives of the program.
Clinical decision support (CDS) is a major component of the new stage 2 rules, which require that eligible professionals who order more than 100 diagnostic imaging procedures during a reporting period must order more than 30% of them using CDS integrated with computerized physician order-entry (CPOE) systems. This acknowledges the federal government's recognition that use of evidence-based guidelines can help reduce the number of inappropriately ordered diagnostic imaging exams.
The good news of 2012 is that those in stage 1 have a year longer to meet the 15 core objectives and five of 10 menu requirements (many of which can be exempted) before moving to stage 2. Even better news is that RIS vendors en masse have certified software. And the rule requiring radiologists to purchase software modules that will never be utilized has been shelved with the 2014 edition of RIS products.
Federal officials reported in early December that almost two-thirds of eligible healthcare professionals have attested to meaningful use, as well as 82% of U.S. hospitals. Billions of dollars have been paid, although it is not possible from government statistics to determine the collective sum that has gone to radiologists.
What was very apparent was that in 2012 federal policymakers realized that one size does not fit all in determining meaningful use for general practice physicians and medical specialties. The progress made this year will make meaningful use much less onerous from radiology's perspective in 2013.
By Cynthia E. Keen
No. 6: Controversy erupts over CT lung cancer screening guidelines
Be careful what you wish for -- you might end up with something completely different. At the beginning of 2012, imaging advocates were excited about the prospect of new guidelines for CT lung cancer screening, the first to reflect recent research showing a drop in mortality after screening with low-dose chest CT.
Unfortunately, the first guidelines to become publicly available set a very high bar, limiting screening exams to the heaviest smokers. As one might expect, controversy followed.
The guidelines were released in May by a joint task force of the American College of Chest Physicians (ACCP) and the American Society of Clinical Oncology (ASCO). They took a modest approach, recommending screening only for smokers and former smokers 55 to 74 years old with a history of at least 30 pack-years.
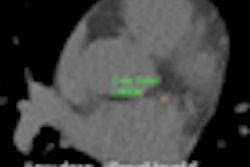
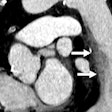
 Screening CT of a 74-year-old woman with a 48 pack-year history of smoking. Nodule is seen at ultralow-dose CT (0.2 mSv) using model-based iterative reconstruction (GE Healthcare). Image courtesy of Dr. Ella Kazerooni.
Screening CT of a 74-year-old woman with a 48 pack-year history of smoking. Nodule is seen at ultralow-dose CT (0.2 mSv) using model-based iterative reconstruction (GE Healthcare). Image courtesy of Dr. Ella Kazerooni.This was the same cohort for which the 2011 National Lung Screening Trial (NLST) had found a 20% mortality benefit. That study offered hope that CT lung cancer screening could finally be the tool to find early-stage cancers in time to cure them.
But screening proponents cried foul over the ACCP/ASCO guidelines, which recommended exclusion of other high-risk individuals, such as patients with chronic obstructive pulmonary disease. They believe that the broader body of research favors a more expansive screening effort.
It's not just a semantic difference. The U.S. government is already wrestling with whether and how to add CT lung cancer screening to the already overburdened Medicare program, and what seem to be minor changes in eligibility criteria could translate into millions of dollars in additional healthcare spending. The debate is also being faced by healthcare systems and third-party payors as they begin to implement their own screening programs.
With that standoff in place, movement toward implementing widespread screening of high-risk individuals has remained slow and controversial. The NLST findings raised questions that are now being studied and debated, primarily related to the frequency of screening, the most appropriate cohort, costs, and treatment options. At least a few centers are laying the groundwork for high-volume screening.
In 2013, look for greater reliance on software tools such as automated volumetric nodule measurement and computer-aided detection to boost accuracy and ease the workload of radiologists, and more use of iterative reconstruction to reduce radiation dose from repeat screening scans. Standardized reporting is on the way, and radiologists will keep trying to distinguish benign from malignant nodules, as well as look for innovative ways to predict which individuals make the best screening candidates.
By Eric Barnes
No. 7: RT hepatitis case spotlights cracks in health worker regulation
When traveling radiologic technologist (RT) David Kwiatkowski was arrested in July 2012 on suspicion of infecting patients with hepatitis C, few observers had any idea how far the case would extend. Five months later, hundreds of patients in a half-dozen states have been tested for hepatitis C, and health authorities are seriously questioning the adequacy of the nation's regulation of allied health personnel.
Kwiatkowski was arrested after a mysterious outbreak of hepatitis C at Exeter Hospital, a 100-bed community hospital in New Hampshire. Several dozen patients who had been seen in the hospital's cardiac catheterization department later developed the same strain of hepatitis C -- a strain that was traced to Kwiatkowski.
 David Kwiatkowski was arrested on suspicion of infecting patients with hepatitis C.
David Kwiatkowski was arrested on suspicion of infecting patients with hepatitis C.The story continued to unfold after Kwiatkowski's arrest as the full extent of his travels became known. As a traveling technologist, he had worked over the years in stints at hospitals in Michigan, Kansas, Pennsylvania, Arizona, and Maryland, among other states.
It was later revealed that he'd been fired from several of these assignments, in some cases after being found in compromising situations with drugs -- typically fentanyl, used to sedate cardiac cath patients -- at his side. Kwiatkowski is suspected of stealing fentanyl for his own use, then putting syringes contaminated with hepatitis C back into the hospital's drug supply.
Kwiatkowski was indicted on December 3 at a federal court in New Hampshire and charged with multiple counts of tampering with a consumer product and obtaining controlled substances by fraud. He faces up to 10 years in prison for each tampering count, and up to four years in prison for each controlled-substances count, as well as a fine. He pleaded not guilty.
As Kwiatkowski's case works its way through the legal system, healthcare providers and regulators are left wondering what went wrong. Kwiatkowski's ability to slip through the profession's cracks -- he never even had his RT license suspended until this year's arrest -- highlights the need for stronger control and oversight of medications in hospitals, as well as a more comprehensive database and enforcement system for healthcare personnel.
Whether such a system is implemented in 2013 remains to be seen.
By Brian Casey