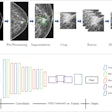
Software developer RadLogics has resolved the warning letter it received from the U.S. Food and Drug Administration (FDA) in 2018 regarding its AlphaPoint software.
In a letter dated October 29 to RadLogics CEO Moshe Becker, the FDA said that it has completed an evaluation of the company's corrective actions and that it appears that the violations in the warning letter have been addressed.