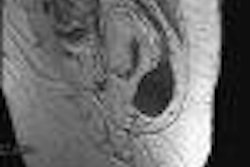
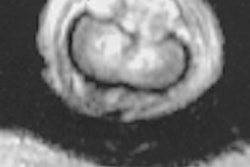
Surgi-Vision of Columbia, MD has received the Food and Drug Administration's 501(k) clearance for the Intercept-Vascular guidewire, an MRI coil that can be threaded into a patient’s artery or vein in order to detect early signs of vascular disease.
The disposable device measures 30/1000 of an inch in diameter, and is attached to a standard MRI scanner. The guidewire can obtain extremely high-resolution, cross-sectional images without exposing the physician or the patient to ionizing radiation or contrast dye, according to the company.
Surgi-Vision will team up with GE Medical Systems to distribute the Intercept-Vascular guidewire and other internal MR products.
By AuntMinnie.com staff writersFebruary 6, 2001
Related Reading
Surgi-Vision gets FDA clearance for internal MRI coil, December 4, 2000
Surgi-Vision wins clearance for MRI coil, October 26, 2000
GE signs deal with Surgi-Vision, March 7, 2000
Copyright © 2001 AuntMinnie.com