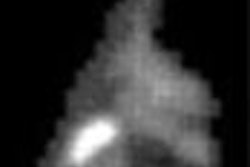
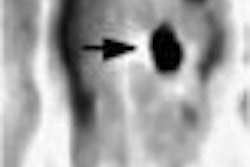
NEW YORK CITY - GE Medical Systems has laid claim to the high end of the burgeoning fusion imaging market, launching its Discovery LS hybrid CT/PET scanner yesterday at a media event at the Waldorf Astoria hotel.
First showcased as a work-in-progress at the 2000 RSNA meeting, Discovery LS combines GE's flagship LightSpeed Plus CT scanner with the Waukesha, WI-based firm's Advance NXi PET scanner. GE executives say the integration of these premium anatomical and functional imaging modalities will usher in a new era of cancer detection and treatment.
"Physicians can tell immediately if the patient has cancer, and know precisely where it is located in the body," said Beth Klein, GE's vice president and general manager of global functional imaging. "They're also able to determine the exact volume of the lesion, and this is critically important for radiation planning. Physicians tell us [Discovery LS] will help them detect cancer earlier, stage it more accurately, and treat it more precisely. That in turn leads to better patient outcomes, shorter hospital stays, and better, more personalized patient care."
The traditional diagnostic work-up process for lung cancer, for example, can last four to six weeks, causing significant patient anxiety, Klein said. But with Discovery LS, GE believes that most of that process can be condensed into a 30-minute noninvasive exam.
Discovery LS has been installed at three beta sites: University Hospital in Zurich, Switzerland; Johns Hopkins Medical Institutions in Baltimore; and Rambam Medical Center in Haifa, Israel. Clinical users were on hand in New York City to discuss their experiences with Discovery LS.
The combination of PET and CT yields a measurable clinical impact, said Dr. Gustav von Schulthess, Ph.D., professor and director of the division of nuclear medicine at the University Hospital of Zurich. Of the first 53 tumor patients scanned using Discovery LS, 25 had lung cancer. With PET alone, 13 patients would have been categorized incorrectly, while with Discovery LS, only 3 would have.
The hospital reported that 60% of tumor manifestations were more accurately located, with a 40% increase in confidence in the location of the lesions.
"This translates into better-tailored therapy for patients," von Schulthess said.
Diagnostic confidence was improved in one of two patients, and critical aspects of diagnosis were improved in one of four patients using Discovery LS, von Schulthess said.
With the recent development of cancer-blocking drugs, hybrid CT/PET technology becomes even more useful, said Dr. Homer Macapinlac, director and section chief of PET at the University of Texas M.D. Anderson Cancer Center in Houston.
"We'd like to personalize cancer care; we want to improve accuracy and effectiveness of these treatments, and then identify the cancers so these patients can get the proper treatment they need, and [we can] monitor the response to their treatments," he said.
GE has high hopes for Discovery LS, expecting 30 shipments by the end of the year. The firm had already received an order by Thursday morning on its Web site, Klein said. The vendor also anticipates that the system will open the door to increased proliferation of PET technology.
"It takes the powerful technology of PET that's existed for 15 to 20 years and really brings it into the mainstream of imaging, [allowing] more patients to experience it," said GE president and chairman-elect Jeffrey Immelt.
Discovery LS will cost approximately $2.7 million. That's a stiff price, but given current reimbursement schedules, institutions will be able to break even economically with a scan volume of approximately five patients a day, according to Joseph Hogan, president and CEO of GE Medical Systems.
Current owners of LightSpeed Plus and Advance NXi will be able to upgrade to Discovery LS. GE has invested $30 million since 1997 in the development of Discovery LS, plus another $80 million for research and development of the LightSpeed CT and Advance NXi scanners.
By Erik L. Ridley
AuntMinnie.com staff writer
June 22, 2001
Related Reading
FDA clears GE combo PET/CT scanner, April 16, 2001
GE and Siemens cross swords in hybrid PET/CT imaging, November 28, 2000
Copyright © 2001 AuntMinnie.com