Image analysis software developer Perspectum has received funding from the U.S. Food and Drug Administration (FDA) Center for Drug Evaluation and Research to speed up the qualification of its cT1 imaging biomarker as a liver disease drug development tool.
The funding will support a study at Massachusetts General Hospital to accelerate the availability of noninvasive tools to diagnose and monitor fatty liver disease. Currently, nonalcoholic steatohepatitis (NASH) is most commonly assessed through liver biopsy, which is invasive and precludes some patients from care.
The FDA provided a $250,000 financial assistance award to support the liver imaging project, noted Perspectum. The company also said the award demonstrates the FDA's recognition of the value of its imaging biomarkers technology.
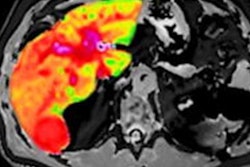
Perspectum has developed LiverMultiScan, a noninvasive tool to evaluate and monitor NASH. LiverMultiScan uses multiparametric MRI-based technology to show correlates of fat, iron, and fibroinflammatory disease in the liver.