HeartLung has received 501(k) clearance from the U.S. Food and Drug Administration (FDA) for its AI-CVD platform.
The platform allows for automated extraction of clinically relevant cardiovascular and systemic measurements from existing chest and abdominal CT scans. This also does not require additional imaging, radiation, contrast, or workflow disruption, the company highlighted.
HeartLung said AI-CVD can be applied to nearly 40 million CT scans performed annually in the U.S. and up to 80 million scans when including head and extremity imaging.
Using AI-CVD quantitative imaging measurements and clinical evaluation, healthcare providers can assess patients who are unaware of their risk of several diseases and conditions, such as coronary heart disease, heart failure, atrial fibrillation, stroke, and liver steatosis.
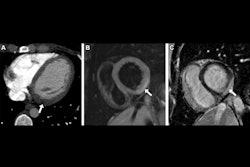
The platform also includes FDA-cleared modules for coronary artery calcium scoring, aortic wall and valve calcium, mitral valve calcium, cardiac chamber volumetry, lung attenuation analysis, and more.
All volumetric measurements are adjusted for body surface area and reported in absolute values and population-based percentiles, referenced to the Multi-Ethnic Study of Atherosclerosis (MESA) and Framingham Heart Study (FHS).