An early analysis of a nine-month period of billing conducted by a healthcare consulting firm determined that chest x-ray CAD is reimbursed by 52 national, regional, and local payors representing over 60 million covered lives.
The sampling of payors included Blue Cross Blue Shield (Florida, Texas, and Utah), Cigna, Federal Blue Cross Blue Shield, Great West National, IHC Care and IHC SelectMed, and Medicare (Railroad), as well as Tricare and Valuecare, according to HealthCare Market Strategies of Yamhill, OR.
"The quantity and mix of payors is important," HealthCare Market Strategies consultant Michael Longacre told AuntMinnie.com. "Many smaller payors will yield to the extensive investigations of larger payors. I have (also) seen scenarios where the extensive coverage by smaller regional payors put pressure on the larger payors to ultimately reimburse for the technology."
In October 2005, CPT III Code 0152T became the first radiological Category III CPT code to have a published non-Medicare resource-based relative value scale (RBRVS) code made available at the time of implementation (January 1, 2006). Although the wording of the code is written to be applicable for any type of use of computer-assisted detection (CAD) software to analyze a digital or digitized chest x-ray, current reimbursement charges today are for the use of lung cancer detection software only.
Private-payor reimbursement has followed within months. Currently, the majority of healthcare providers have adopted a protocol of requesting reimbursement of one CAD procedure every six months for repeat patients, even though the lung CAD software may be utilized routinely with each chest x-ray exam, Longacre said.
"Users who charge $27 for Code 0152T receive reimbursement between $10 and the full amount of $27," Longacre said. "In one case study, over a period of nine months, a provider collected 31% of charges and charged off 30%, leaving 29% still to be adjudicated."
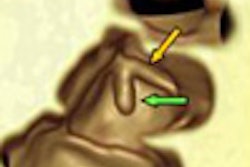
Introduced at the 2004 RSNA meeting by Riverain Medical of Miamisburg, OH (following early development by Deus Technologies), and cleared by the U.S. Food and Drug Administration (FDA), the firm's lung CAD offering is designed to detect suspicious lesions of 9 mm to 3 cm in size on chest x-rays. In April 2006, Philips Medical Systems of Andover, MA, introduced similar FDA-cleared software licensed from EDDA Technology of Princeton, NJ, designed to detect lesions of 5 mm to 2 cm.
Lung CAD software to analyze chest x-rays is designed to identify areas for additional evaluation by a radiologist after an initial interpretation has been made. Adoption and utilization has been slower than mammography CAD, however, as mammography CAD is used with a cancer screening radiographic procedure; lung CAD software is employed with the radiographic images of patients presenting with other symptoms.
In the U.S., chest CT and not chest x-ray is the gold standard for diagnosing a patient presenting with symptoms of lung cancer. Lung CAD software for chest radiographs is designed to detect nodules representing early stages of lung cancer that might be visible in a chest radiograph taken for other diagnostic purposes.
Many radiologists are awaiting the publication of more clinical studies that show the utility of CAD before incorporating the technology, according to CAD researchers Dr. Heber MacMahon, professor of radiology and section chief of thoracic radiology at University of Chicago Hospitals in Illinois, and Dr. Edwin van Beek, Ph.D., professor of radiology at University of Iowa Hospitals and Clinics in Iowa City.
In association with their colleagues, MacMahon and van Beek are authoring scientific papers and posters on this topic at the upcoming 2006 RSNA meeting in Chicago.
The other impediment was the fact that the first iterations of both Riverain's RapidScreen and EDDA's IQQA-Chest software were offered only on standalone workstations that were not integrated with PACS. Users like Dr. C. Mark Alder, a solo radiologist serving the 55-physician multispecialty practice of Ogden Clinic in Ogden, UT, say that the use of lung CAD software adds about a minute to the interpretation of a routine chest x-ray. The radiology department of Ogden Clinic is digitizing analog chest x-rays until a PACS is installed.
At Utah Imaging Associates in Bountiful, UT, the 10 radiologists of the practice waited until the software could be fully integrated with its PACS and the PACS of Davis Hospital and Medical Center in Layton, UT, according to chief operating officer Brett Palmer.
"They wanted to be able to have the processed images accessible with the chest study," Palmer said.
The University of Iowa Hospitals and Clinics, a research and beta site for EDDA Technology, also waited to formally purchase the software until it could be integrated with its PACS, which has now happened.
"Residents and fellows are finding the software to be an excellent aid," van Beek observed. "They have about a 20% higher detection rate of nodules compared to not using lung CAD detection software."
Van Beek said that the University of Iowa Hospitals and Clinics plans to start charging payors soon.
By Cynthia E. Keen
AuntMinnie.com contributing writer
October 24, 2006
Disclosure notice: Ms. Keen occasionally performs consulting work with several medical imaging vendors.
Related Reading
X-ray CAD aids in early lung cancer detection, June 22, 2006
NCI CAD database program gathers momentum, March 16, 2006
Chest x-ray CAD reaches reimbursement milestone, November 22, 2005
X-ray CAD finds bone defects in rheumatoid arthritis, May 18, 2004
Copyright © 2006 AuntMinnie.com