Breast biopsy device developer TransMed7 said that commercial production versions of its Concorde US ultrasound-guided soft-tissue biopsy devices have been used in a series of clinical cases.
Dr. Michael Berry used Concorde US to successfully perform breast biopsy cases at the Margaret West Comprehensive Breast Center in Germantown, TN, according to TransMed7.
One of two models in TransMed7's Concorde breast biopsy platform, Concorde US is designed for either ultrasound guidance for handheld use or with an optional and reusable stage mount adapter for stereotactic and 2D/3D tomosynthesis use, accordion to the firm. Meanwhile, Concorde ST is also suitable for stereotactic and 2D/3D tomosynthesis-guided breast biopsy procedures and offers console-replacing capabilities, TransMed7 said.
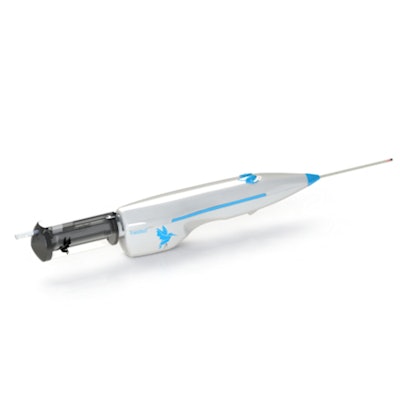
 Concorde US ultrasound-guided soft-tissue biopsy device equipped with the company's detachable VacuPac device for tetherless operation. Image courtesy of TransMed7.
Concorde US ultrasound-guided soft-tissue biopsy device equipped with the company's detachable VacuPac device for tetherless operation. Image courtesy of TransMed7.Both models are automated, vacuum-assisted, 10-14-gauge devices that selectively provide forward coring or combined forward and shielded side coring, as well as full fluid management and the firm's tissue-shielding technology at the working end of the coring needle, according to the company.
The Concorde devices are based on TransMed7's Zero5 work element, which is composed of a fused, single element constructed from three hypotubes. This element is then laser-cut and welded to form articulable twin cutter blades at the end of a rotating open tube, with the addition of an outer scoopula shield, according to the vendor. It penetrates, cores, severs, and provides a pathway to transport multiple tissue samples into a detachable chamber via a closed-circuit fluid management system and vacuum system, the company said.
TransMed7 plans to launch the Concorde devices in the first quarter.