Contrast agent developer Palatin Technologies of Cranbury, NJ, and corporate partner Mallinckrodt have received approval from the Food and Drug Administration to market and distribute NeutroSpec.
The product, formerly known as LeuTech in clinical trials, is an imaging agent designed to help diagnose cases of appendicitis in patients five years and older with atypical symptoms, according to St. Louis-based Mallinckrodt.
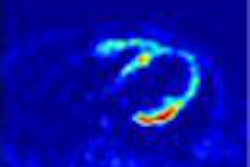
NeutroSpec is a radiolabeled monoclonal antibody -- technetium (99m-Tc) fanolesomab -- that binds to white blood cells, which localize at infection sites. When injected into a patient's bloodstream, NeutroSpec radiolabels white blood cells and myeloid precursors with a radioactive tracer. A physician is then able to locate the site of infection with images generated by a gamma camera, Mallinckrodt said.
Although the product is currently approved only for use in detecting difficult-to-diagnose appendicitis, Mallinckrodt said that phase II clinical trials are under way to determine the efficacy of the product in diagnosing osteomyelitis as well as the imaging and detection of other infections, such as fever of unknown origin, postsurgical abscesses, and inflammatory bowel disease.
By AuntMinnie.com staff writersJuly 6, 2004
Related Reading
Palatin nets $22.7 million, January 30, 2004
Palatin raises $19 million, April 1, 2003
Mallinckrodt gets FDA nod for MR contrast power injection, March 18, 2003
Mallinckrodt adds to contrast injector line, December 1, 2002
Palatin, Mallinckrodt restructure deal, May 15, 2002
Copyright © 2004 AuntMinnie.com