While strokes caused by carotid artery stenosis are preventable, duplex ultrasound screening has failed to demonstrate cost-effectiveness. The developers of an inexpensive ultrasound device hope to change the equation with a two-step screening process analogous to the practice of mammography.
A team led by researchers from ultrasound technology developer DVX has determined that carotid artery stenosis screening can be cost-effectively performed in the primary care setting by nurses using an inexpensive, continuous-wave vector ultrasound Doppler system. Similar to the practice of screening mammography, patients with suspicious findings -- in this case, peak systolic blood velocities greater than 140 cm/sec -- would be referred for a duplex ultrasound for diagnosis.
If this two-step method was broadly adopted, stroke could be reduced and billions of dollars saved within the U.S. healthcare system, according to Dr. David Vilkomerson, president of Princeton, NJ-based DVX. He presented the group's findings during a session at the American Institute of Ultrasound in Medicine (AIUM) meeting in San Diego.
Preventing stroke
Approximately half of the 700,000 strokes each year in the U.S. are caused by plaque in the region of the carotid bifurcation. Previous research has also shown that carotid-caused stroke is preventable; operating on ultrasound-detected stenoses of greater than 60% led to a significantly reduced stroke rate (11% to 3.8%) over five years.
However, duplex ultrasound screening for significant carotid stenoses has not been proved cost-effective, adding a cost of $80,000 per quality-adjusted life year (QALY). With a prevalence of significant stenosis of around 5% and a procedure cost of $210 for a duplex ultrasound screening study, even in an older population it costs $4,200 to find one significant stenosis, Vilkomerson said.
Believing there was a better way, Vilkomerson and colleagues proposed an initial screening step prior to color-flow duplex ultrasound, eyeing a screening test that would be performed in the physician's office to increase the percentage of positive findings for those eventually receiving duplex studies.
To be cost-effective, the first screening step must be inexpensive to perform, relying on nonspecialist operators and a low-cost instrument. It also must take a short amount of time, Vilkomerson said.
Working off the principle that the highest blood velocity in the neck leads to the carotid bifurcation, there's no need for imaging, he said. The Doppler sound signal can be used to find and follow the carotids, with systolic blood velocities greater than 140 cm/sec indicating stenosis of more than 50%. And because there's no anatomy to interpret, there's also no requirement for a trained sonographer.
Funded by a Small Business Innovation Research (SBIR) grant program from the U.S. National Institutes of Health's National Heart, Lung, and Blood Institute, DVX developed an ultrasound system that utilizes a scanhead with six receivers around a central transmitter. Designed for carotid scans, the scanhead covers the location of more than 95% of carotid bifurcations.
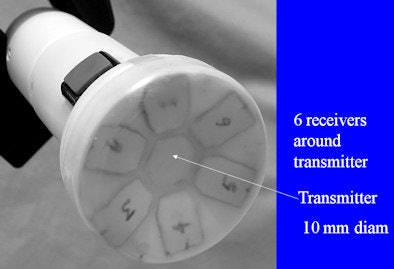 |
| Scanhead. All images courtesy of Dr. David Vilkomerson. |
It's placed flat on the skin and is orientation-independent; operation is fully automatic, Vilkomerson said. Doppler audio is specially processed to accentuate indications of stenosis, and the system records raw digital data and retrospectively processes it to provide automatic operation and velocity calculation.
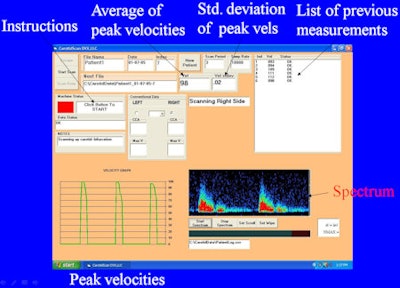 |
| Instrument display. |
Of course, this approach can generate false positives, Vilkomerson said.
"Every external carotid stenosis is not clinically significant," he said. "Tortuous vessels, funny curves will give you false positives, but that's part of the deal."
To test the system, a group of primary care doctors in the Princeton, NJ, area participating in the Carotid Artery Screening System Investigators (CASSI) group invited their patients older than 65 for screening if they had at least one of the following risk factors: hypertension, heart disease, dyslipidemia, and a history of smoking.
Some patients were examined in the office, while others were referred to a central screening location. All patients were examined by nurses from the physicians' offices who had just one to two hours of training on the instrument, Vilkomerson said.
Of the 898 patients screened from July 2008 to December 2009, 23% were referred to duplex ultrasound; 16% of the referrals were then found to have greater than 50% stenoses. The screening exam took an average of 4.3 minutes ± 1.8.
Based on the nurse cost of $50 per hour, a 15-minute exam, and the equipment cost, the screening exam would cost less than $15, he said. As a result, the total cost of finding a greater than 50% stenosis is $1,530, compared with $4,200 when only performing duplex ultrasound screening.
Delving further into the cost-effectiveness issue, the researchers evaluated the potential cost of screening for 1,000 patients based on the CASSI trial findings:
- First screening step cost: 1,000 x $15 = $15,000
- Second screening step cost: 230 (23% referral rate) x $210 = $48,300
- 29 stenoses > 60% that received carotid endarterectomy (CEA) at $5,300 = $154,000
- Total screen and treat cost = $219,000
However, the treated patients would have their stroke rate reduced by 7%, averting 2.1 strokes in the patient population of 1,000. Given that stroke is known to cost the healthcare system $190,000, that's a savings of $392,000.
"That's net savings of $172,000," Vilkomerson said. "That doesn't sound like a lot until you look at all of the Medicare patients, 70% of whom fit the profile of our screening. That would represent [savings of] $4.2 billion. And that's not counting quality of life, the nonfinancial costs of having a stroke victim in your family."
DVX has not yet submitted an application for the device to the U.S. Food and Drug Administration (FDA), pending a large clinical trial, Vilkomerson said.
By Erik L. Ridley
AuntMinnie.com staff writer
April 9, 2010
Related Reading
How to navigate pitfalls in carotid ultrasound, September 9, 2009
How to provide great service in the ultrasound vascular lab, July 22, 2009
Future of ultrasound will require technology, educational gains, April 4, 2009
B-mode US shines in measuring carotid atherosclerosis, September 19, 2008
Vascular ultrasound QA requires consistency, validation, August 4, 2008
Copyright © 2010 AuntMinnie.com