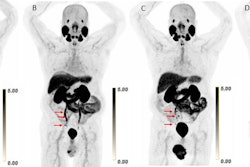
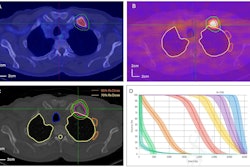
Subtle differences in how prostate-specific membrane antigen (PSMA) PET radiotracers perform in patients prior to Pluvicto treatment may determine which ones become the future tracers of choice, according to a group in Germany.
Researchers led by Jan Heilinger, MD, of University Hospital Cologne, compared the use of gallium-68 (Ga-68) PSMA-11 and F-18 DCFPyl -- two U.S.-approved PET imaging agents -- in a small group of patients. The tracers were equal in defining "PSMA-positivity" (an eligibility requirement for Pluvicto treatment), yet F-18 DCFPyl PET resulted in better image quality, the group found.
"Significant differences were observed in terms of contrast-to-noise ratios, thereby demonstrating the better image quality obtained with F-18 DCFPyL-PET," the group wrote, in an article published September 20 in EJNMMI Research.
Ga-68 PSMA-11 was approved for prostate cancer imaging by the U.S. Food and Drug Administration (FDA) in 2020, followed by F-18 DCFPyL (Pylarify) in 2021. Both tracers are highly effective for imaging prostate cancer. Last year, the FDA approved Pluvicto (Lu-177 PSMA-617) for treating the disease, with PET-positivity used as a benchmark to determine which patients are eligible for the therapy.
However, the two tracers may differ in uptake behavior, and their comparability with regard to patient selection for Pluvicto therapy has not yet been established, the group noted.
In a step toward that, the group studied differences in the two approaches in a group of 11 patients who underwent PET scans using both tracers in short succession as part of their clinical workup between July 2014 and December 2016. A total of 24 tumor lesions were identified in both PET scans, including 15 lymph node metastases, six local recurrences, and three bone metastases.
According to a quantitative software analysis, the researchers observed no differences between Ga-68 PSMA-11 PET and F-18 DCFPyL PET in tumor-to-liver ratios, meaning all of the patients would qualify for Pluvicto therapy.
However, a highly significant difference was observed between the PET tracers in contrast-to-noise ratios, they found. Specifically, the median contrast-to-noise ratio was 10.7 on Ga-68 PSMA-11 PET versus 21.6 on F-18 DCFPyL PET, according to the results.
"Overall, contrast-to-noise ratios were higher on F-18 DCFPyL-PET compared to those on Ga-68 PSMA-11 PET," the group wrote.
Ultimately, contrast-to-noise ratios signify the ability of PET imaging to differentiate between cancer cells and healthy tissue. While these ratios aren't clinical measures used to diagnose disease, higher ratios can improve the "detectability" of tumors, the researchers wrote.
Moreover, PSMA ligands labeled with F-18 isotopes are produced in larger volumes in cyclotron facilities, while Ga-68-based ligands are produced in smaller batches using generators in hospital nuclear medicine departments, the authors noted.
"Given their advantages, F-18-labeled PSMA ligands could become the future tracers of choice in PET diagnostics, as F-18 labeling makes onsite radiolabeling unnecessary and would enable wider commercial distribution," the group concluded.
The full article is available here.