Doctors now have a reliable approach for determining whether prostate cancer patients are responding to prostate cancer treatment with Pluvicto, according to research published July 11 in Radiology.
U.S. and European researchers led by first author Andrei Gafita, MD, of the University of California, Los Angeles tested a novel framework called the Response Evaluation Criteria in Prostate-specific Membrane Antigen (PSMA) PET/CT (RECIP 1.0) and found its proposed quantitative measurements for patient response matched with visual reads of imaging by radiologists.
"Qualitatively assessed RECIP demonstrated excellent agreement with quantitative RECIP and excellent interreader reliability and can be readily implemented in clinical practice for response evaluation in men with metastatic castration-resistant prostate cancer undergoing Lu-177 PSMA therapy," the group wrote.
Pluvicto (lutetium-177 [Lu-177] prostate-specific membrane antigen [PSMA]-617) is a radioligand therapy approved by the U.S. Food and Drug Administration in March 2022. The drug is indicated for adult patients with metastatic castration-resistant prostate cancer and is among the first of a newer class of treatments known as theranostics (therapy combined with diagnostics) to receive approval for any disease, with others in development.
Also last year, prior to the treatment's approval, researchers began developing RECIP 1.0 for evaluating how patients respond to the drug. The framework uses results of PET/CT scans using PSMA radiotracers, which indicate how severe prostate cancer may be, or whether it has decreased, based on uptake of the tracers by tumors.
In this study, the group aimed to establish the RECIP 1.0 framework further by assessing the agreement between tumor segmentation software (quantitative RECIP) and qualitative reads by nuclear medicine physicians (visual RECIP).
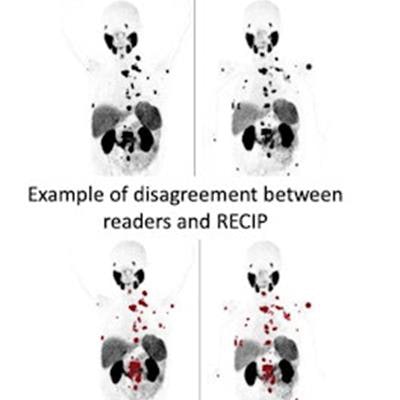
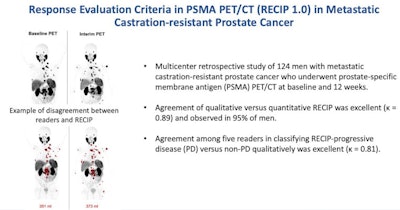 Image courtesy of Radiology.
Image courtesy of Radiology.The study was conducted at three academic centers and included 124 men who received Pluvicto treatment between December 2014 and July 2019. PSMA PET/CT images at baseline and 12 weeks were assessed qualitatively by five readers for changes in total tumor volume and for new lesions. The primary outcomes were the agreement between visual and quantitative RECIP and the interreader reliability of visual RECIP. Forty (32%) of the patients had quantitative RECIP progressive disease and 84 (68%) of the men had nonprogressive disease.
According to the findings, the agreement between visual versus quantitative RECIP was achieved in 118 of 124 men (95%) and resulted in an excellent Fleiss' kappa (k) value of 0.89, the researchers wrote. Agreement among readers in classifying visual RECIP progressive disease versus nonprogressive disease was also excellent (k = 0.81; 103 of 124 men, or 83%).
In addition, RECIP progressive disease was associated with significantly shorter overall survival compared with nonprogressive disease (hazard ratio, 2.6), the researchers added.
"[RECIP 1.0] can be readily implemented in clinical practice for response evaluation in men with metastatic castration-resistant prostate cancer undergoing Lu-177 PSMA therapy," the group concluded.
The full study can be found here.