NEW ORLEANS – Re-treating prostate cancer patients with lutetium-177 radioligand therapy after they have completed their initial course appears to improve overall survival, according to a study presented June 21 at the Society of Nuclear Medicine and Molecular Imaging 2025 annual meeting.
Gokce Bilgin, MD, of the Mayo Clinic in Rochester, MN, presented research showing that a second course of lutetium-177 (Lu-177) prostate-specific membrane antigen-617 (PSMA-617) resulted in a median overall survival of 14.5 months.
 Gokce Bilgin, MD, presented results of a Pluvicto study June 21 at SNMMI 2025 in New Orleans.
Gokce Bilgin, MD, presented results of a Pluvicto study June 21 at SNMMI 2025 in New Orleans.
“For patients who respond to treatment and experience progression only after completing their initial course of therapy, re-treatment with Lu-177 PSMA-617 has emerged as a potential approach,” she said, during a young investigator award symposium.
Radioligand therapy (RLT) with Lu-PSMA-617 (Pluvicto, Novartis) has shown significant benefits in patients with metastatic castration-resistant prostate cancer (mCRPC), including prolonged overall survival and progression-free survival, as well as improved quality of life, Bilgin explained. Lu-PSMA-617 was initially approved in the U.S. in 2022, and researchers continue to explore expanded uses for the therapy.
In this study, Bilgin and colleagues analyzed clinical and imaging data of patients who underwent at least two cycles of the therapy, followed by a second course of treatment between March 2022 and December 2024. Out of a total of 589 patients, they identified 20 (3.4%) who received RLT re-treatment.
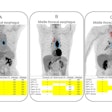
Following the completion of their initial treatment, re-treatment consisted of a median of four cycles after a mean interval of 28.8 ± 17.9 months. A prostate-specific antigen (PSA) decline of ≥ 50% was observed in nine out of the 20 patients (45%), including three who achieved a > 90% reduction. Among these responders, seven maintained their biochemical response at a three-month follow-up, and six sustained it at a six-month follow-up.
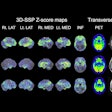
A favorable imaging response was observed in 10 of the patients (50%), with four achieving a complete response and six achieving a partial response, whereas 10 patients (50%) showed progression. The median PSA-progression-free survival was nine months (range: 1.4–28).
Lastly, the median overall survival was 46.8 months (range: 16.9–78.5) from the initial treatment and 14.5 months (range: 1.4–27.6) following re-treatment, Bilgin reported.
She added that grade 2 renal toxicity was observed in one patient. One patient experienced grade 1 thrombocytopenia, while three patients developed grade 2 anemia. Notably, no grade 3 or grade 4 adverse events were observed during either treatment period, Bilgin said.
“While mild toxicities appeared more frequently during pretreatment, preexisting marrow and renal compromise may contribute to toxicity in this heavily pretreated population, rather than a direct effect of Lu-177 PSMA-617 alone,” she said.
Ultimately, re-treatment with Lu-177 PSMA-617 RLT resulted in a significant PSA response and demonstrated encouraging survival outcomes, Bilgin noted. However, given the small sample size, further investigation in larger populations is warranted, she said, and added that prospective trials are underway both in Europe and the U.S. to evaluate re-treatment outcomes, namely the RE-LuPSMA and ReaLup trials.