F-18 fluoroestradiol (FES)-PET scans can predict whether or not endocrine therapy may be effective in women with estrogen receptor (ER)-positive breast cancer, according to a study published March 13 in the Journal of Nuclear Medicine.
The finding is from a meta-analysis of published studies and further supports the use of the technique to improve outcomes for patients, noted lead author Ashwin Singh Parihar, MD, of the Mallinckrodt Institute of Radiology in St. Louis, MO, and colleagues.
“F-18 FES-PET is a successful biomarker for predicting the likelihood of success of endocrine therapy in patients with ER-positive breast cancer,” the group wrote.
Selecting patients with ER-positive breast cancer for endocrine therapy is typically based on biopsy results, the authors explained. Yet this method doesn’t account for tumor heterogeneity, or cellular differences between tumors that affect how they respond to treatment, the authors explained.
Conversely, F-18 FES-PET is a noninvasive, whole-body molecular imaging technique approved in 2020 that captures both spatial (differences based on location), and, when ER-positive patients are imaged at multiple time points, temporal heterogeneity (how tumors change over time), the authors noted.
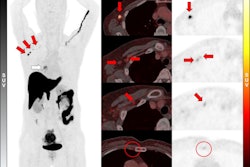
To consolidate evidence on the value of the technique, the researchers analyzed 12 published studies up to November 1, 2024, that included 308 participants with data on baseline F-18 FES-PET scans and their subsequent response to endocrine therapy. They estimated the likely benefit from endocrine therapy after positive and negative F-18 FES PET scans, as well as the risk ratio of clinical benefit compared between the two.
 A visual abstract of the analysis. Image courtesy of the Journal of Nuclear Medicine.
A visual abstract of the analysis. Image courtesy of the Journal of Nuclear Medicine.
According to the analysis, the likelihood of clinical benefit after a positive F-18 FES-PET scan was 66% (n = 227 participants) and the likelihood after a negative scan was 11% (n = 81 participants).
In addition, the risk ratio of response that compared those who were F-18 FES-positive with those who were F-18 FES-negative was 3.21 (p < 0.0001), meaning those whose tumors were positive were about three times more likely to respond to treatment than those with negative scans, the researchers reported.
“There is strong evidence that patients with F-18 FES-negative disease are unlikely to respond to endocrine therapies, despite the presence of ER-positive disease on pathology, which may relate to the significant disease heterogeneity,” the researchers wrote.
Ultimately, ER-positive breast cancer represents a large subset of the disease, and endocrine therapies remain foundational in treatment, the researchers noted. However, they wrote that accurately predicting which patients will derive benefit from these therapies is critical for optimizing outcomes.
In this regard, F-18 FES-PET has a potential role in helping personalize treatment strategies, the researchers suggested.
“This [analysis] supports a role for F-18 FES-PET in identifying patients for whom endocrine therapy may or may not be an appropriate treatment option,” they concluded.
The full study is available here.