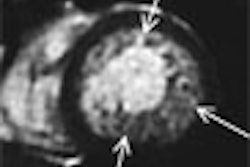
Coronary imaging developer InfraReDx reported that the U.S. Food and Drug Administration has issued 510(k) clearance for the company to market its near-infrared (NIR) spectroscopic system for examining coronary arteries.
The InfraReDx intracoronary spectroscopy system is a fiber-optic, catheter-based device with a laser light source. The catheter is designed to perform spectroscopy of motion and blood flow in the coronary arteries, according to the Burlington, MA-based company.
By AuntMinnie.com staff writers
October 19, 2006
Related Reading
Near-infrared spectroscopy can identify vulnerable plaques in atherosclerosis, September 29, 2002
Copyright © 2006 AuntMinnie.com