The U.S. Food and Drug Administration has signed off on a premarket approval (PMA) application from Siemens Medical Solutions for its syngo Lung computer-aided detection (CAD) software for assisting radiologists in the detection of solid lung nodules in CT chest exams.
The Malvern, PA-based company noted that syngo Lung was validated in a multicenter, multireader study of approximately 200 cases reviewed by 17 radiologists using data from four U.S. medical centers.
syngo Lung CAD is second-generation nodule detection software from Siemens, following syngo LungCARE NEV (nodule-enhanced viewing), which was introduced in December 2003 and has more than 500 installations worldwide.
By AuntMinnie.com staff writers
October 20, 2006
Related Reading
Siemens debuts 3D medical imaging display, October 19, 2006
Siemens signs Catholic Healthcare Partners, October 17, 2006
Siemens aligns with Access e-forms, October 10, 2006
Siemens unveils healthcare budgeting tool, October 5, 2006
Siemens, Komen Foundation partner, October 2, 2006
Copyright © 2006 AuntMinnie.com



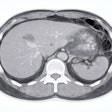
![Images show the pectoralis muscles of a healthy male individual who never smoked (age, 66 years; height, 178 cm; body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], 28.4; number of cigarette pack-years, 0; forced expiratory volume in 1 second [FEV1], 97.6% predicted; FEV1: forced vital capacity [FVC] ratio, 0.71; pectoralis muscle area [PMA], 59.4 cm2; pectoralis muscle volume [PMV], 764 cm3) and a male individual with a smoking history and chronic obstructive pulmonary disorder (COPD) (age, 66 years; height, 178 cm; BMI, 27.5; number of cigarette pack-years, 43.2, FEV1, 48% predicted; FEV1:FVC, 0.56; PMA, 35 cm2; PMV, 480.8 cm3) from the Canadian Cohort Obstructive Lung Disease (i.e., CanCOLD) study. The CT image is shown in the axial plane. The PMV is automatically extracted using the developed deep learning model and overlayed onto the lungs for visual clarity.](https://img.auntminnie.com/mindful/smg/workspaces/default/uploads/2026/03/genkin.25LqljVF0y.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&h=100&q=70&w=100)


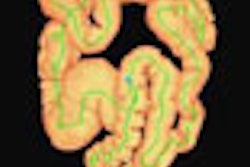
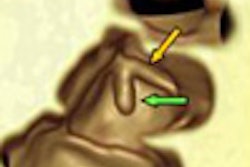


![Images show the pectoralis muscles of a healthy male individual who never smoked (age, 66 years; height, 178 cm; body mass index [BMI, calculated as weight in kilograms divided by height in meters squared], 28.4; number of cigarette pack-years, 0; forced expiratory volume in 1 second [FEV1], 97.6% predicted; FEV1: forced vital capacity [FVC] ratio, 0.71; pectoralis muscle area [PMA], 59.4 cm2; pectoralis muscle volume [PMV], 764 cm3) and a male individual with a smoking history and chronic obstructive pulmonary disorder (COPD) (age, 66 years; height, 178 cm; BMI, 27.5; number of cigarette pack-years, 43.2, FEV1, 48% predicted; FEV1:FVC, 0.56; PMA, 35 cm2; PMV, 480.8 cm3) from the Canadian Cohort Obstructive Lung Disease (i.e., CanCOLD) study. The CT image is shown in the axial plane. The PMV is automatically extracted using the developed deep learning model and overlayed onto the lungs for visual clarity.](https://img.auntminnie.com/mindful/smg/workspaces/default/uploads/2026/03/genkin.25LqljVF0y.jpg?auto=format%2Ccompress&crop=focalpoint&fit=crop&h=112&q=70&w=112)