Philips and Polarean have launched the next phase of their partnership to increase access to xenon-based lung ventilation imaging for patients with obstructive lung diseases.
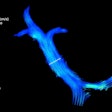
The emerging technique uses hyperpolarized xenon gas as a signaling agent, which allows clinicians to see how air flows through different parts of the lungs in real time. Xenon MRI is approved in the U.S. for evaluating lung ventilation in adults and children ages 12 and older and is now pending approval for children as young as 6, Philips noted.
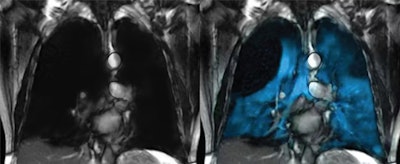 Xenoview. Image courtesy of Philips.
Xenoview. Image courtesy of Philips.
The technology is comprised of Polarean’s Xenoview 3-tesla chest coil, which is integrated into Philips' clinical workflow across 3-tesla MRI systems. The scan requires just two 10-second breath holds and can be completed in under a minute, the company added.
The companies established the partnership in June 2023 and are featuring the technology this week at the International Society for Magnetic Resonance in Medicine 2025 meeting in Honolulu, HI.