A new PET tracer can accurately detect and differentiate a certain type of kidney cancer from other kidney tumor types, according to research presented June 26 at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual meeting.
In his presentation, Jeremie Calais, MD, from the University of California, Los Angeles discussed his team's findings, which found that its zirconium-89 (Zr-89)-based PET tracer delivered high performance in a study examining clear cell renal carcinoma.
"[Imaging with the tracer] has the potential to be practice-changing by guiding patient management, for example, in the selection of surgical versus conservative management and in helping avoid unnecessary biopsies or surgeries and the associated risks and costs," Calais said.
Renal masses are detected using CT, ultrasound, or biopsy. However, the researchers pointed out that these methods are "insufficient." Structural imaging cannot separate benign from malignant masses, while biopsy is invasive, prone to sampling errors, and is unable to detect extrarenal disease, according to the authors.
In an effort to help, Calais and colleagues developed their new PET tracer, named Zr-89-DFO-girentuximab, from a monoclonal antibody called girentuximab. It was created to target carbonic anhydrase IX (CAIX), an enzyme prevalent in clear cell renal carcinoma.
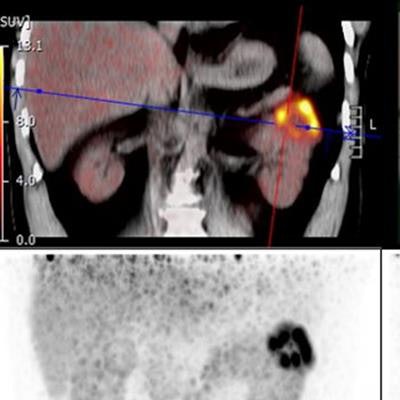
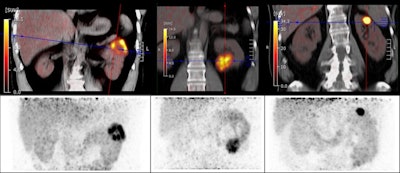 CAIX-targeted PET/CT with Zr-89-DFO girentuximab allows for the visualization and characterization of renal masses with great image contrast, according to a study presented at the SNMMI annual meeting. Researchers said that the tracer shows high uptake in clear cell renal carcinoma lesions and low background activity in the normal renal parenchyma and other normal organs. Images courtesy of the SNMMI.
CAIX-targeted PET/CT with Zr-89-DFO girentuximab allows for the visualization and characterization of renal masses with great image contrast, according to a study presented at the SNMMI annual meeting. Researchers said that the tracer shows high uptake in clear cell renal carcinoma lesions and low background activity in the normal renal parenchyma and other normal organs. Images courtesy of the SNMMI.The team wanted to test the tracer in PET/CT imaging to detect this type of kidney cancer through the international Zircon study, an open-label, multicenter clinical trial. They included data from 34 recruited patients who had an undefined kidney mass and planned surgery. The patients were administered a single dose of the tracer followed by a PET/CT scan five days later. From there, the patients underwent a nephrectomy, and renal mass tissue was collected to determine a final histology. All tumors were cT1 with tumor size ranging from 1.5 cm to 6.7 cm.
The researchers found that of the total patients, 23 were diagnosed with clear cell renal cell carcinoma. The remaining 11 patients were diagnosed with oncocytomas, chromophobe renal cell carcinoma, collecting duct tumor, malignant sarcoma, and renal cell carcinoma with tuberous sclerosis complex features.
Additionally, the researchers reported that Zr-89-DFO-girentuximab was read as positive in 20 out of the 23 clear cell renal cell carcinoma patients, and all benign and non-clear cell renal cell carcinomas were interpreted as negative on the scan. The team also found a perfect positive predictive value (100%) for Zr-89-DFO-girentuximab PET.
Of 12 clear cell renal carcinoma tumors with loss of the chromosome 3p, 11 were reported as positive, while 10 of 10 tumors with positive CAIX expression by immunohistochemistry were also reported as positive by the scan.
Calais said that based on their results, imaging with the tracer could support earlier diagnosis. This in turn could reduce emotional burden on patients, "especially" those in active surveillance. Calais also suggested that the tracer could have potential in whole-body distant staging, post-treatment surveillance, and treatment monitoring, as well as theranostic applications.
The researchers indicated that they are working with an expanded access program to launch the tracer. Here, the U.S. Food and Drug Administration works with companies to allow access to investigational therapies outside of a clinical trial to patients with serious or life-threatening illnesses. For these patients, there are no comparable or satisfactory alternate therapies.
If approved, the program will allow the radiopharmaceutical to be made available for use to describe clear cell renal carcinomas previously identified with CT or MRI, according to the researchers.