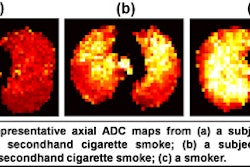
Toshiba America Medical Systems has received U.S. Food and Drug Administration clearance for its open-bore 1.5-tesla Vantage Titan MR scanner.
The Tustin, CA-based vendor said the company is on schedule to release the system in the first quarter.
Related Reading
Toshiba basks in Aquilion One glow, but more work remains, December 24, 2007
Toshiba, TomTec to partner on cardiac 4D ultrasound, December 6, 2007
Toshiba adds to its education initiative, December 3, 2007
Vital Images, Toshiba ink distribution deal, November 27, 2007
AquilionONE 320-slice CT scanner spearheads Toshiba RSNA launches, November 26, 2007
Copyright © 2008 AuntMinnie.com