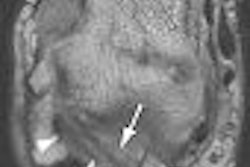
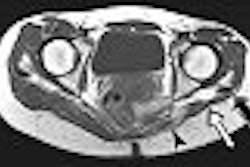
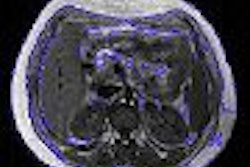
MRI contrast developer Epix Pharmaceuticals said that the U.S. Food and Drug Administration (FDA) has denied its formal appeal to approve its Vasovist blood-pool imaging agent. The FDA also turned down Epix's request for an advisory committee to review Vasovist, according to the Lexington, MA-based firm.
In its response letter, the FDA's Office of New Drugs (OND) also suggested that if Epix decided to conduct additional clinical research to support approval, a safer course of action would be to conduct two new clinical trials, according to Epix. Epix president Dr. Andrew Uprichard said the company is evaluating several options, including an appeal to the next level at the FDA.
By AuntMinnie.com staff writers
August 28, 2006
Related Reading
Epix, Predix complete merger, August 17, 2006
Epix, Predix shareholders approve merger, August 15, 2006
Epix nears Australia approval for Vasovist, August 14, 2006
Epix grows revenues in Q2, August 7, 2006
FDA extends Epix's Vasovist appeal, July 31, 2006
Copyright © 2006 AuntMinnie.com