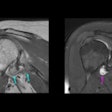
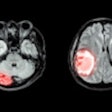
The U.S. Food and Drug Administration (FDA) has approved the compatibility of InSightec's Exablate Neuro device with Siemens Healthineers' Magnetom Skyra, Prisma, and PrismaFit MRI scanners to treat patients with essential tremor.
Exablate Neuro uses MR-guided focused ultrasound to target and ablate tissue deep within the brain with no incisions. It is FDA-cleared to treat medication-refractory essential tremor. Clinical trials are also underway to evaluate potential applications in the treatment of various neurological diseases.