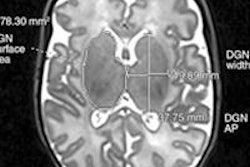
The U.S. Food and Drug Administration (FDA) has approved a new indication for Bayer HealthCare's Gadavist (gadobutrol) MRI contrast agent.
Gadavist has been cleared for intravenous use with breast MRI to assess the presence and extent of malignant breast disease. The approval is based on priority review of two multicenter, phase III studies (GEMMA-1 and GEMMA-2) conducted in 13 countries, Bayer said.