Women's healthcare vendor Hologic of Bedford, MA, has received a date for review of its Selenia Dimensions 3D digital mammography tomosynthesis system by the U.S. Food and Drug Administration (FDA).
The review is part of Hologic's premarket approval application (PMA) and will be held by the FDA's Radiological Devices Panel on September 24.
Hologic originally filed its PMA for the system in 2008. It seeks clearance of Selenia Dimensions for both screening and diagnostic applications; Hologic is also conducting additional clinical trials for a separate FDA submission it plans to file later.
In the U.S., Selenia Dimensions is available as a 2D system that is upgradeable to perform 3D breast tomosynthesis imaging if and when the product is approved by the FDA. The device is currently available for 3D use in Europe, the Middle East, South America, Asia, Australia, Canada, and Mexico.
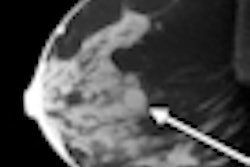
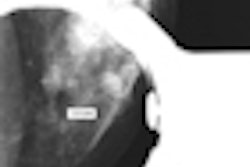
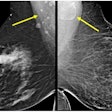
The system is designed to address a basic limitation of 2D digital mammography: the superposition of normal breast anatomy that may mask a breast cancer, according to Hologic.
Related Reading
Hologic to buy breast MR firm Sentinelle for $85M, July 6, 2010
Hologic's Selenia nets EUREF certification, June 23, 2010
Hologic posts Q2 revenue gain, May 4, 2010
Hologic debuts upright biopsy at ECR, March 8, 2010
Hologic, Ethicon bury the hatchet, February 22, 2010
Copyright © 2010 AuntMinnie.com