Full-field digital mammography (FFDM) units now make up more than 50% of the installed base of mammography systems in the U.S., according to data from the U.S. Food and Drug Administration (FDA).
The FDA said that as of April 1, 2009, 50.3% of the mammography systems certified under the Mammography Quality Standards Act (MQSA) were FFDM units, with 6,577 in use. This compares to a 15% penetration level for FFDM systems in December 2006. The number of certified facilities with one or more FFDM unit reached 4,371, which is 49.9% of certified facilities, the agency said.
The FDA noted that the first FFDM unit was approved for marketing in January 2000, and there are currently 10 different FFDM products that have received FDA approval.
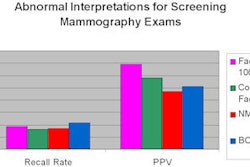
The agency added that dose measurements performed as part of MQSA requirements show that FFDM units have overall lower average glandular doses compared to film-screen units. Additionally, phantom image scores are generally higher for FFDM units.
Related Reading
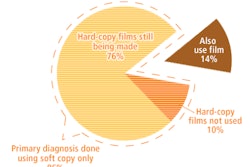
FFDM equals analog mammography in hard-copy reading, May 7, 2009
Digital mammography transition: The agony and the ecstasy, May 5, 2009
Mammography procedure volume drops 16% since 2000, March 17, 2009
FFDM performs well versus analog for screening, studies show, January 6, 2009
Copyright © 2009 AuntMinnie.com