Interest in PET imaging for cancer applications has increased over the past five years as the Health Care Financing Administration (HCFA) has continued to add these procedures to its reimbursement rolls. But breast cancer imaging hasn’t yet joined the ranks of covered applications.
In light of the medical community’s interest in PET and other breast cancer imaging technologies, a new company, Positron Emission Mammography (PEM) Technologies of Bethesda, MD, has begun developing PET scanners specifically for breast imaging. With PET’s potential for improved specificity, sensitivity, and tumor delineation, PEM Technologies believes that PET breast imaging will become increasingly attractive to both HCFA and to doctors, particularly surgeons, who could use it for surgical planning.
"We hope that as we present material to HCFA we’ll have a good chance at coverage. Molecular imaging is where the industry is going, and PET is a good fit for that," said Dr. Irving Weinberg, present and CEO of PEM Technologies.
As a radiology resident and clinical fellow at the Johns Hopkins Hospital, Weinberg, who is also a nuclear medicine physician and a physicist, was struck by what he saw as x-ray technology’s lack of specificity. In 1993, he developed the concept of what would become the company’s core technology: a real-time, high-resolution PET camera. Two years later, he founded PEM Technologies.
"At Johns Hopkins, I watched mammographers make (clinical) decisions with limited information," Weinberg said. "I saw a need for breast imaging equipment that had more specificity and sensitivity. We’ve developed a PET scanner that attaches to traditional mammography units, as well as a compression device for gamma cameras. We’re also developing handheld PET and gamma cameras for breast and perhaps prostate applications."
PET technology isn’t optimized for applications like breast imaging, according to Weinberg, since it doesn’t yet have the resolution and collection efficiency to image small breast lesions. But the company’s PET camera is intended to make the use of PET possible for smaller imaging applications, and for both diagnosis and surgical planning.
The scanner comes in two sizes: one with two-inch cube detectors for intraoperative applications, and one with six-inch cube detectors for full-breast imaging. It can be used with conventional mammography units or as a stand-alone device, and gathers image data 50 times faster than a conventional PET camera, allowing for real-time feedback from biopsies and taking between 30 seconds and four minutes to produce an image, according to the company.
PEM Technologies has used Lorad mammography units with its scanner, which has not yet been cleared by the FDA, Weinberg said. Last year the company sold one of the scanners on an investigational basis to Wake Forest University-Baptist Medical Center in Winston-Salem, NC. The company expects to price the smaller scanner at approximately $150,000, and the larger scanner for $300,000.
In addition to its scanner, PEM Technologies has been developing a compression device for use with scintimammography machines. On Feb. 8, the company received clearance from the Food and Drug Administration for its compression device, which supports, positions, and immobilizes the breast during the exam. The unit performs the same functions as an x-ray compression device, including the ability to rapidly release compression and to perform automated compression and automated release with hand compression.
The compression device is being used at Harbor-UCLA Medical Center in Torrance, CA, in a clinical trial that is exploring the sensitivity of breast imaging radiopharmaceuticals. The company may apply for FDA clearance for biopsy capability of the compression device, according to Weinberg.
"If you compress the breast, you get a more controlled exam. By bringing the breast closer to the camera with our compression device, resolution improves, and technicians are also able to perform biopsies. If you can’t do biopsies (with a mammography device), you’re in trouble," he said.
The company is banking its business plan on the positive results coming from clinical trials of breast PET imaging, which point to potential benefits over x-ray mammography or scintimammography.
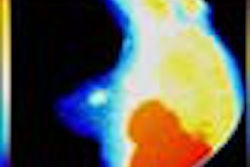
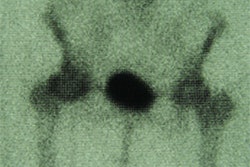
"X-ray mammography doesn’t detect cancer; it detects non-fatty tissue," Weinberg said. "In younger patients with a lot of functional breast tissue, it’s harder to find breast cancer. But PET images the biological activity of tissue, which should make its sensitivity and specificity better than x-ray. PET imaging may also be useful in surgical planning: We’ve seen things on the PEM images that we couldn’t see from conventional mammography images, like the size of the cancer and the multifocality of the cancer in the same breast, which are important to surgeons. And PET is faster than scintimammography."
PEM Technologies hopes to establish partnerships with OEMs rather than selling its products directly to the market, and may eventually expand its offerings to include prostate cancer applications.
By Kate Madden YeeAuntMinnie.com contributing writer
March 14, 2001
Related Reading
PEM gets FDA nod for breast imaging device, February 14, 2001
Auto spot compression mammography could aid imaging of dense breast tissue, January 30, 2001
Positron emission mammography shows promise, January 5, 2001
HCFA broadens Medicare coverage for PET, December 18, 2000
Click here to post your comments about this story. Please include the headline of the article in your message.
Copyright © 2001 AuntMinnie.com