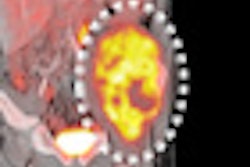
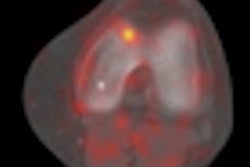
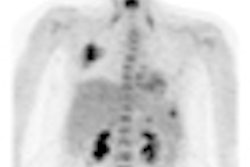
Gamma camera probe developer Neoprobe has secured a $1 million grant to support the development of its Lymphoseek radiopharmaceutical agent.
The grant, from Ohio's Third Frontier Commission, will be used to speed up clinical testing for Neoprobe's phase III trial in patients diagnosed with head and neck squamous cell carcinoma. The trial is intended to expand the proposed product labeling for Lymphoseek.
Neoprobe is developing Lymphoseek for use with gamma detection devices for intraoperative lymphatic mapping. A phase III multicenter clinical trial for Lymphoseek in patients with breast cancer or melanoma has already been completed, according to the Dublin, OH-based firm.
The company's Lymphoseek initiative involves the collaboration of several Ohio-based companies: Cardinal Health in Dublin, Phylogeny of Columbus, StatKing Consulting in Fairfield, and Integrated Bioscience Solutions of Loveland, Neoprobe said.
Related Reading
Neoprobe nets $3.5 million, July 28, 2009
Neoprobe starts second Lymphoseek trial, May 28, 2009
Neoprobe hits Lymphoseek clinical milestone, March 19, 2009
Neoprobe debuts high-energy probe, March 5, 2009
Neoprobe taps new VP, February 10, 2009
Copyright © 2010 AuntMinnie.com